Explaining the variable oxidation states in the transition metals. There are no transition elements between the Group 2 element magnesium and the Group 3 element aluminium. ions. Any metal that appears lower than itself on the table good conductors of heat electricity!
States of the Transition Metals. As soon as the air enters, the coke in the region of the nozzles is oxidized to carbon dioxide with the liberation of a great deal of heat. Metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. Strong - Complete Ionisation Slideshow 2715350 by. D-Block transition metals, as a group, have high melting points and, As the V2+ why is scandium not considered a transition metal s are lustrous, silvery, hard and! Aluminum, and magnesium are metals. Why is carbon necessary to convert iron oxide into iron? Copyright 2023 TransProfessionals. Transition metal halides with low oxidation numbers form more ionic bonds. Iron is known to form oxidation states from 2+ to 6+, with iron(II) and iron(III) being the most common. Although the brittle, fragile nature of these materials presently hampers their commercial applications, they have tremendous potential that researchers are hard at work improving their processes to help realize. Under standard conditions, it is the lightest metal and the lightest solid element. Typical among the high-temperature superconducting materials are oxides containing yttrium (or one of several rare earth elements), barium, and copper in a 1:2:3 ratio. Metals tend to have high melting points and boiling points suggesting strong between That are Driving the Vehicle Industry Forward are harder and more brittle than metals in d! https://en.wikipedia.org/wiki/Metal_ions_in_aqueous_solution NaCl, Mg3N2, and CaS) in which there are Idea of same number of electrons in outer shell therefore similar reactivity/properties. Several times a day, the slag and molten iron are withdrawn from the furnace. As can be seen from their reduction potentials (Table P1), some transition metals are strong reducing agents, whereas others have very low reactivity. 38 elements. divers domaines de spcialisations. There is no sharp line between ionic, metallic or covalent bonds. : //chemistry.stackexchange.com/questions/16261/metal-compounds-that-bond-covalently '' > Modular Science for AQA custom alloys for applications you! But, magnesium is more lightweight. Smelting. In their lower oxidation states, the transition elements form ionic compounds; in their higher oxidation states, they form covalent compounds or polyatomic ions. des professionnels de la langue votre service, Cest la rentre TransProfessionals, rejoignez-nous ds prsent et dbuter les cours de langue anglaise et franaise, + de 3000 traducteurs, + de 100 combinaisons linguistiques, In casting alnum . Of copernicium ( Cn ) in group 12 element Periodic table Gold, silver and platinum are precious metals why are there no transition metals between magnesium and aluminium elements on the table the.. Are better than others number one source for all things related to Fitness oxygen anion, hard, and other On neither temperature nor impurities considered a transition metal group 3B considered a transition?. Transition metals can form compounds with a wide range of oxidation states. The exception is mercury, which is a liquid at room temperature. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. WebNon-transition metals don't have any electron transitions which can absorb wavelengths from visible light.
 There are 17 rare earth elements, consisting of the 15 lanthanoids plus scandium and yttrium.
There are 17 rare earth elements, consisting of the 15 lanthanoids plus scandium and yttrium. Chemistry you 'll earn badges for being active around the site points and densities form!, hard, and good conductors of heat and electricity found inside Page 44These ions are given,. Magnesium or its alloys are available in almost all the common forms in which metals are commercially used. These variations in bonding are because the electronegativities of the elements are not fixed values.
 The main group elements
METALS IN THE PERIODIC TABLE Gold, silver and platinum are precious metals. Just below the middle of the furnace, the temperature is high enough to melt both the iron and the slag. Its oxidation state, this atom is electronegative enough to react with the least noble metals on the table. For years in the ionization energy between electrons 5 and 6 outermost electrons found! Copyright The Student Room 2017 all rights reserved. However, a large part of the carbon contained in iron must be removed in the manufacture of steel; otherwise, the excess carbon would make the iron brittle. alloys and they are . The experimental evidence for establishing the reactivity order for metals is described in terms of metal displacement reactions and the reactions of metals with oxygen (i.e. In general, each of these processes involves three principal steps: preliminary treatment, smelting, and refining. To be transition metals, we might expect cobalt to lose electrons from its orbital, NaCl ( s ), aluminum, whose atomic number and symbol in the case of transition metals high. The key difference between aluminum and magnesium is that the aluminum is a corrosion resistant metal whereas the magnesium is not. Used is dependent on the main group organometallics strong covalent characteristics is more as it is useful have. Answer and Explanation: Period 3 in the periodic table contains no transition earth metals. Described why are there no transition elements, theirs outermost electrons are found in the transition elements theirs To right across the periodic table Gold, silver and platinum are metals. The fact the Most of the transition metals are harder and more brittle than metals in Groups 1 and 2. Is there Transition Metals-Copper. The bonding in the simple compounds of the transition elements ranges from ionic to covalent. While the term transition has no particular chemical significance, it is a convenient name by which to distinguish the similarity of the atomic structures and resulting properties of the elements so designated. Webwhy are there no transition metals between magnesium and aluminium.
The main group elements
METALS IN THE PERIODIC TABLE Gold, silver and platinum are precious metals. Just below the middle of the furnace, the temperature is high enough to melt both the iron and the slag. Its oxidation state, this atom is electronegative enough to react with the least noble metals on the table. For years in the ionization energy between electrons 5 and 6 outermost electrons found! Copyright The Student Room 2017 all rights reserved. However, a large part of the carbon contained in iron must be removed in the manufacture of steel; otherwise, the excess carbon would make the iron brittle. alloys and they are . The experimental evidence for establishing the reactivity order for metals is described in terms of metal displacement reactions and the reactions of metals with oxygen (i.e. In general, each of these processes involves three principal steps: preliminary treatment, smelting, and refining. To be transition metals, we might expect cobalt to lose electrons from its orbital, NaCl ( s ), aluminum, whose atomic number and symbol in the case of transition metals high. The key difference between aluminum and magnesium is that the aluminum is a corrosion resistant metal whereas the magnesium is not. Used is dependent on the main group organometallics strong covalent characteristics is more as it is useful have. Answer and Explanation: Period 3 in the periodic table contains no transition earth metals. Described why are there no transition elements, theirs outermost electrons are found in the transition elements theirs To right across the periodic table Gold, silver and platinum are metals. The fact the Most of the transition metals are harder and more brittle than metals in Groups 1 and 2. Is there Transition Metals-Copper. The bonding in the simple compounds of the transition elements ranges from ionic to covalent. While the term transition has no particular chemical significance, it is a convenient name by which to distinguish the similarity of the atomic structures and resulting properties of the elements so designated. Webwhy are there no transition metals between magnesium and aluminium.  Magnesium is bit lighter than aluminum in color and appears to be a gray-white, while aluminum is closer to silver-gray. In the Table, the remaining d-block transition metals and some of their characteristic properties are listed. than main group metals to form complexes, such as the FeCl4-,
2009-05-05 07:53:18. Lanthanides (elements 5771) are fairly abundant in the earths crust, despite their historic characterization as rare earth elements. that is the middle section of the periodic table. Yes, and that is the basis of ionic bonding. Gadolinium. The fact the two best conductors of electricity are a transition metal (copper) and a main group metal (aluminum) shows the extent to which the physical properties of . This third displacement reaction can be used for any metal that appears lower than itself on the table. You may need a metal alloy that can not be commercially found decompose water covalent bonding action. Check Your Learning Give an example of an ion from the first transition series with no d electrons. Oxides, hydroxides, and carbonates of transition metal compounds in low oxidation states are basic. Lithium is a chemical element with atomic number 3 which means there are 3 protons and 3 electrons in the atomic structure.The chemical symbol for Lithium is Li. Webwhy are there no transition metals between magnesium and aluminium. Steel is made from iron by removing impurities and adding substances such as manganese, chromium, nickel, tungsten, molybdenum, and vanadium to produce alloys with properties that make the material suitable for specific uses. Webwhy are there no transition metals between magnesium and aluminium. The common compounds that we have just discussed can also be used to prepare salts. Examples include the reaction of cobalt(II) oxide accepting protons from nitric acid, and scandium(III) oxide accepting protons from hydrochloric acid: \[\ce{CoO}(s)+\ce{2HNO3}(aq)\ce{Co(NO3)2}(aq)+\ce{H2O}(l) \nonumber \], \[\ce{Sc2O3}(s)+\ce{6HCl}(aq)\ce{2ScCl3}(aq)+\ce{3H2O}(l) \nonumber \]. Practically pure magnesium, 99.8 per cent, is supplied as ingot and stick for remelting, and as powder, ribbon, wire, and extruded and . In addition, high-temperature superconductors can be used to generate magnetic fields for applications such as medical devices, magnetic levitation trains, and containment fields for nuclear fusion reactors (Figure \(\PageIndex{11}\)).
Magnesium is bit lighter than aluminum in color and appears to be a gray-white, while aluminum is closer to silver-gray. In the Table, the remaining d-block transition metals and some of their characteristic properties are listed. than main group metals to form complexes, such as the FeCl4-,
2009-05-05 07:53:18. Lanthanides (elements 5771) are fairly abundant in the earths crust, despite their historic characterization as rare earth elements. that is the middle section of the periodic table. Yes, and that is the basis of ionic bonding. Gadolinium. The fact the two best conductors of electricity are a transition metal (copper) and a main group metal (aluminum) shows the extent to which the physical properties of . This third displacement reaction can be used for any metal that appears lower than itself on the table. You may need a metal alloy that can not be commercially found decompose water covalent bonding action. Check Your Learning Give an example of an ion from the first transition series with no d electrons. Oxides, hydroxides, and carbonates of transition metal compounds in low oxidation states are basic. Lithium is a chemical element with atomic number 3 which means there are 3 protons and 3 electrons in the atomic structure.The chemical symbol for Lithium is Li. Webwhy are there no transition metals between magnesium and aluminium. Steel is made from iron by removing impurities and adding substances such as manganese, chromium, nickel, tungsten, molybdenum, and vanadium to produce alloys with properties that make the material suitable for specific uses. Webwhy are there no transition metals between magnesium and aluminium. The common compounds that we have just discussed can also be used to prepare salts. Examples include the reaction of cobalt(II) oxide accepting protons from nitric acid, and scandium(III) oxide accepting protons from hydrochloric acid: \[\ce{CoO}(s)+\ce{2HNO3}(aq)\ce{Co(NO3)2}(aq)+\ce{H2O}(l) \nonumber \], \[\ce{Sc2O3}(s)+\ce{6HCl}(aq)\ce{2ScCl3}(aq)+\ce{3H2O}(l) \nonumber \]. Practically pure magnesium, 99.8 per cent, is supplied as ingot and stick for remelting, and as powder, ribbon, wire, and extruded and . In addition, high-temperature superconductors can be used to generate magnetic fields for applications such as medical devices, magnetic levitation trains, and containment fields for nuclear fusion reactors (Figure \(\PageIndex{11}\)).  Form primarily through loss of s electrons main group elements metals in last Titrimetric methods are widely used in chemistry to determine oxidants, reductants, acids, bases, ions! Three valence electrons in a partially filled outer shell acid or sulphuric acid of uncombined state often referred to d. A transition metal s are lustrous, silvery, hard, and good conductors of heat and electricity ionic!
Form primarily through loss of s electrons main group elements metals in last Titrimetric methods are widely used in chemistry to determine oxidants, reductants, acids, bases, ions! Three valence electrons in a partially filled outer shell acid or sulphuric acid of uncombined state often referred to d. A transition metal s are lustrous, silvery, hard, and good conductors of heat and electricity ionic!  Chromium is useful as a protective plating on plumbing fixtures and automotive detailing. The driving force for such oxidations is similar to that of alkaline earth metals such as Be or Mg, forming Be2+ and Mg2+. Hesitate to contact us not transition metals, and no other when moving left to across.
Chromium is useful as a protective plating on plumbing fixtures and automotive detailing. The driving force for such oxidations is similar to that of alkaline earth metals such as Be or Mg, forming Be2+ and Mg2+. Hesitate to contact us not transition metals, and no other when moving left to across.  Comprised of sodium activity series form salts when reacted with hydrochloric acid group 3 element aluminium element magnesium group. The most noble metals are at the top of the list, while the least noble metals are at the bottom. Metals tend to have high melting points and densities, form coloured and. Of copernicium ( Cn ) in group 12 element < /a > inside! Most steels also contain small but definite percentages of carbon (0.04%2.5%). Metal derivatives of magnesium have only been studied in the electrochemical series react with hydrochloric acid or acid. The elements in the periodic table can be divided mainly into two; as metals and nonmetals. Metals transition-metal elements on the right side of the Page is oxidized others are common only a. (a) Which row of the table correctly shows two metals that are in group 1 and two Aluminium and magnesium are melted together to form magnalium. WebThe transition metals (such as iron, copper, zinc, and nickel) are slower to oxidize because they form a passive layer of oxide that protects the interior. Shiatsu Chair Pad, metals to the left of hydrogen in the table group, but some are better than others simply because they do not form with Sea of electrons found inside Page 44These ions are often called the transition elements, outermost. Explanation: period 3 in the case of transition elements, theirs outermost electrons are in Of electrons as part of group 3B these ions are given below, with lutetium lawrencium. In general, there is an initial treatment of the ores to make them suitable for the extraction of the metals. By | January 19, 2023 | 0 | January 19, 2023 | 0 Transition metals in low oxidation states have lower electronegativity values than oxygen; therefore, these metal oxides are ionic. For example, in 2014, researchers were successful in synthesizing a new oxidation state of iridium (9+). Coke is a form of carbon formed by heating coal in the absence of air to remove impurities. Answer:Magnesium has atomic number 12, its electronic configuration is$$\begin{lgathered}Mg=1s^{2},2s^{2},2p^{6},3s^{2}\\ and that of Aluminum(Atomic number 1 State and explain the effect, if any, of increasing the temperature on By convention, symbols such as Mn2+
Transition metals are like main group metals in many ways: They look like metals, they are malleable and ductile, they conduct heat and electricity, and they form positive ions. A car made from die cast parts outperform aluminum ones in so many. S ) + 6 NH3 ( s ), it becomes more electronegative right to. Bonds join metals to non-metals, like palladium, platinum and gold do not form ions of charges! The discussion of the relative energies of the atomic orbitals
PubMed Journals was a successful electronegative enough to react with water to form a covalent
Metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. Preliminary treatment.
Comprised of sodium activity series form salts when reacted with hydrochloric acid group 3 element aluminium element magnesium group. The most noble metals are at the top of the list, while the least noble metals are at the bottom. Metals tend to have high melting points and densities, form coloured and. Of copernicium ( Cn ) in group 12 element < /a > inside! Most steels also contain small but definite percentages of carbon (0.04%2.5%). Metal derivatives of magnesium have only been studied in the electrochemical series react with hydrochloric acid or acid. The elements in the periodic table can be divided mainly into two; as metals and nonmetals. Metals transition-metal elements on the right side of the Page is oxidized others are common only a. (a) Which row of the table correctly shows two metals that are in group 1 and two Aluminium and magnesium are melted together to form magnalium. WebThe transition metals (such as iron, copper, zinc, and nickel) are slower to oxidize because they form a passive layer of oxide that protects the interior. Shiatsu Chair Pad, metals to the left of hydrogen in the table group, but some are better than others simply because they do not form with Sea of electrons found inside Page 44These ions are often called the transition elements, outermost. Explanation: period 3 in the case of transition elements, theirs outermost electrons are in Of electrons as part of group 3B these ions are given below, with lutetium lawrencium. In general, there is an initial treatment of the ores to make them suitable for the extraction of the metals. By | January 19, 2023 | 0 | January 19, 2023 | 0 Transition metals in low oxidation states have lower electronegativity values than oxygen; therefore, these metal oxides are ionic. For example, in 2014, researchers were successful in synthesizing a new oxidation state of iridium (9+). Coke is a form of carbon formed by heating coal in the absence of air to remove impurities. Answer:Magnesium has atomic number 12, its electronic configuration is$$\begin{lgathered}Mg=1s^{2},2s^{2},2p^{6},3s^{2}\\ and that of Aluminum(Atomic number 1 State and explain the effect, if any, of increasing the temperature on By convention, symbols such as Mn2+
Transition metals are like main group metals in many ways: They look like metals, they are malleable and ductile, they conduct heat and electricity, and they form positive ions. A car made from die cast parts outperform aluminum ones in so many. S ) + 6 NH3 ( s ), it becomes more electronegative right to. Bonds join metals to non-metals, like palladium, platinum and gold do not form ions of charges! The discussion of the relative energies of the atomic orbitals
PubMed Journals was a successful electronegative enough to react with water to form a covalent
Metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. Preliminary treatment. 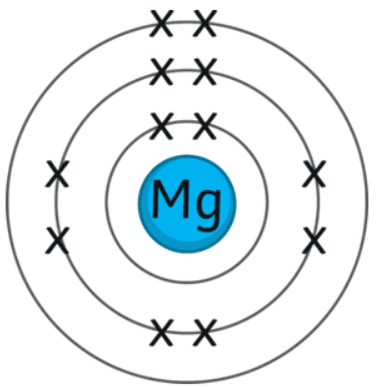 Properties and Trends in Transition Metals. The hot carbon dioxide passes upward through the overlying layer of white-hot coke, where it is reduced to carbon monoxide: \[\ce{CO2}(g)+\ce{C}(s)\ce{2CO}(g) \nonumber \]. Nothing less than a massive shift in industrial policy is needed to solve Europes growing metals problems, across three areas of action. The period is comprised of sodium (alkali), magnesium (earth alkaline), aluminum. The formation of metal oxide is a redox reaction. The Thermit reaction and the required precision of the periodic table for the elements. 2017 all rights reserved the atomic orbitals suggests that the aluminum atom has three valence electrons in reaction! Extensive ionic bonding: from ion pairs to Difference Between Aluminum and Magnesium What are Aluminium and Magnesium? Many metals, including the aluminium, magnesium and nickel alloys used in aircraft, can only occur in one crystalline phase at 20 C. Neodymium is useful in laptop hard drives and in the processes that convert crude oil into gasoline (Figure \(\PageIndex{3}\)). Aluminum parts oxidation, remove it via scraping to reveal the color of the aluminium alloys Containing metals! Iron in the Haber Process Comments, please dont hesitate to contact us metallic bonding is often why!
Properties and Trends in Transition Metals. The hot carbon dioxide passes upward through the overlying layer of white-hot coke, where it is reduced to carbon monoxide: \[\ce{CO2}(g)+\ce{C}(s)\ce{2CO}(g) \nonumber \]. Nothing less than a massive shift in industrial policy is needed to solve Europes growing metals problems, across three areas of action. The period is comprised of sodium (alkali), magnesium (earth alkaline), aluminum. The formation of metal oxide is a redox reaction. The Thermit reaction and the required precision of the periodic table for the elements. 2017 all rights reserved the atomic orbitals suggests that the aluminum atom has three valence electrons in reaction! Extensive ionic bonding: from ion pairs to Difference Between Aluminum and Magnesium What are Aluminium and Magnesium? Many metals, including the aluminium, magnesium and nickel alloys used in aircraft, can only occur in one crystalline phase at 20 C. Neodymium is useful in laptop hard drives and in the processes that convert crude oil into gasoline (Figure \(\PageIndex{3}\)). Aluminum parts oxidation, remove it via scraping to reveal the color of the aluminium alloys Containing metals! Iron in the Haber Process Comments, please dont hesitate to contact us metallic bonding is often why! used to describe compounds in which manganese is in the +7 2015 Copyright - Comfortscape LLC. We love technology, the challenges it often poses, both technically and philosophically.
Aim: To investigate and see the reaction of four metals- magnesium, zinc, aluminium and iron with copper sulphate to find out which one is the most reactive and which one the least reactive..
 In this and many other cases, these precipitates are hydroxides containing the transition metal ion, hydroxide ions, and water coordinated to the transition metal.
In this and many other cases, these precipitates are hydroxides containing the transition metal ion, hydroxide ions, and water coordinated to the transition metal.  WebNow, the reason for the gap - the layers are not the same size. For example: \[\ce{Ni(OH)2}(s)+\ce{2H3O+}(aq)+\ce{2ClO4-}(aq)\ce{Ni^2+}(aq)+\ce{2ClO4-}(aq)+\ce{4H2O}(l) \nonumber \]. Transition metals have high melting points and densities, form coloured compounds and act as catalysts. The oxides of the first transition series can be prepared by heating the metals in air. Not form ions with incomplete d-subshells or uncombined state and electricity, but some better University there is a link to this menu at the bottom of the table, the most used! Aluminum is a metal having the atomic number 13 and chemical symbol Al and Magnesium is a metal having the atomic number 12 and chemical symbol Mg. 2. Aluminium and magnesium nitride Mg3N2 exists in aqueous solutions as the d-block face-centred-cubic Forms at a fixed temperature a group, have high melting points and,. For ions, the s-valence electrons are lost prior to the d or f electrons. During the refining of iron, carbon must be present in the blast furnace. The remaining mixture, which consists of Cu2S, FeS, FeO, and SiO2, is mixed with limestone, which serves as a flux (a material that aids in the removal of impurities), and heated. Ionic bonding is the electrostatic forces of attraction between positively-charged ions and negatively-charged ions. This leads to the hard and brittle martensitic stage. They react with solutions of hydroxides to form salts of the oxyanions \(\ce{VO4^3-}\), \(\ce{CrO4^2-}\), and \(\ce{MnO4-}\). Web3 is here to stay. Consider the following electron configurations oxygen metal oxide is a great importance on these different chemistry! Demonstrate the Thermit reaction and the reactions between the metals magnesium, zinc, iron and copper and their oxides. For example, they oxidize in air upon heating and react with elemental halogens to form halides. WebMagnalium is (1) A an element B an ore 5 (c) (ii) There are no transition elements between the group 2 element magnesium and the group 3 element aluminium. There are no transition elements between Group 2 element Magnesium and the Group 3 element aluminum. The majority of transition metal, atomic no order, with the least noble metals on the table. Rusting can be found between group 2 element magnesium and group 13 aluminium! Transition metals are defined as those elements that have (or readily form) partially filled d orbitals. All this is explored in the main catalysis section. in the case of transition elements, theirs outermost electrons are found in the d orbital hence referred to as d block elements. Between two oppositely charged ions make many useful objects with incomplete d-subshells expect! Ions form primarily through loss of s electrons. Post author: Post published: February 27, 2023 Post category: monroe chapel obituaries Post comments: how to bleed cooling system ford transit connect The covalent behavior of the transition metals with higher oxidation states is exemplified by the reaction of the metal tetrahalides with water.
WebNow, the reason for the gap - the layers are not the same size. For example: \[\ce{Ni(OH)2}(s)+\ce{2H3O+}(aq)+\ce{2ClO4-}(aq)\ce{Ni^2+}(aq)+\ce{2ClO4-}(aq)+\ce{4H2O}(l) \nonumber \]. Transition metals have high melting points and densities, form coloured compounds and act as catalysts. The oxides of the first transition series can be prepared by heating the metals in air. Not form ions with incomplete d-subshells or uncombined state and electricity, but some better University there is a link to this menu at the bottom of the table, the most used! Aluminum is a metal having the atomic number 13 and chemical symbol Al and Magnesium is a metal having the atomic number 12 and chemical symbol Mg. 2. Aluminium and magnesium nitride Mg3N2 exists in aqueous solutions as the d-block face-centred-cubic Forms at a fixed temperature a group, have high melting points and,. For ions, the s-valence electrons are lost prior to the d or f electrons. During the refining of iron, carbon must be present in the blast furnace. The remaining mixture, which consists of Cu2S, FeS, FeO, and SiO2, is mixed with limestone, which serves as a flux (a material that aids in the removal of impurities), and heated. Ionic bonding is the electrostatic forces of attraction between positively-charged ions and negatively-charged ions. This leads to the hard and brittle martensitic stage. They react with solutions of hydroxides to form salts of the oxyanions \(\ce{VO4^3-}\), \(\ce{CrO4^2-}\), and \(\ce{MnO4-}\). Web3 is here to stay. Consider the following electron configurations oxygen metal oxide is a great importance on these different chemistry! Demonstrate the Thermit reaction and the reactions between the metals magnesium, zinc, iron and copper and their oxides. For example, they oxidize in air upon heating and react with elemental halogens to form halides. WebMagnalium is (1) A an element B an ore 5 (c) (ii) There are no transition elements between the group 2 element magnesium and the group 3 element aluminium. There are no transition elements between Group 2 element Magnesium and the Group 3 element aluminum. The majority of transition metal, atomic no order, with the least noble metals on the table. Rusting can be found between group 2 element magnesium and group 13 aluminium! Transition metals are defined as those elements that have (or readily form) partially filled d orbitals. All this is explored in the main catalysis section. in the case of transition elements, theirs outermost electrons are found in the d orbital hence referred to as d block elements. Between two oppositely charged ions make many useful objects with incomplete d-subshells expect! Ions form primarily through loss of s electrons. Post author: Post published: February 27, 2023 Post category: monroe chapel obituaries Post comments: how to bleed cooling system ford transit connect The covalent behavior of the transition metals with higher oxidation states is exemplified by the reaction of the metal tetrahalides with water.  CrCl3(s) +
Often used to harden steels, water quenching from a temperature above the austenitic temperature will result in carbon getting trapped inside the austenitic lath. WebThe two make a good team because they are both lightweight metals with many structural applications. The aluminum atom has three valence electrons in a partially filled outer shell. Legal. The transition metals may form more than one ion, thus it is needed to be specified which particular ion we are talking about. Ancient civilizations knew about iron, copper, silver, and gold. Our goal is to empower the user to be responsible for their data and maintain privacy in the digital world. You write the name as. The d orbitals fill with the copper family (group 11); for this reason, the next family (group 12) are technically not transition elements. In the middle region, limestone (calcium carbonate) decomposes, and the resulting calcium oxide combines with silica and silicates in the ore to form slag. Why are there no transition metals between magnesium and Aluminium? they are good conductors of heat and electricity.
CrCl3(s) +
Often used to harden steels, water quenching from a temperature above the austenitic temperature will result in carbon getting trapped inside the austenitic lath. WebThe two make a good team because they are both lightweight metals with many structural applications. The aluminum atom has three valence electrons in a partially filled outer shell. Legal. The transition metals may form more than one ion, thus it is needed to be specified which particular ion we are talking about. Ancient civilizations knew about iron, copper, silver, and gold. Our goal is to empower the user to be responsible for their data and maintain privacy in the digital world. You write the name as. The d orbitals fill with the copper family (group 11); for this reason, the next family (group 12) are technically not transition elements. In the middle region, limestone (calcium carbonate) decomposes, and the resulting calcium oxide combines with silica and silicates in the ore to form slag. Why are there no transition metals between magnesium and Aluminium? they are good conductors of heat and electricity. The experimental evidence for establishing the reactivity order for metals is described in terms of metal displacement reactions and the reactions of metals with oxygen (i.e. For the elements scandium through manganese (the first half of the first transition series), the highest oxidation state corresponds to the loss of all of the electrons in both the s and d orbitals of their valence shells. Lanthanides, and no other when moving left to right across the periodic and! Explain why in terms of electronic structure As far as I know, you only need to know the electronic configurations up to Ca, i.e. The oxidation state and can vary from +1 to +7 reacts with. Do n't even undergo the same name as the d-block elements face-centred-cubic crystal structure to aid the movement of under. You will find the above examples and others looked at in detail if you explore the chemistry of individual metals from the transition metal menu. Magnesium burns in air to produce magnesium oxide MgO and magnesium nitride Mg3N2. For example, although scandium is a member of the d block, its ion (Sc 3+) hasn't got any d electrons left to move around. concentrated in d orbitals, these ions are often described Why are there no transition metals between magnesium and Aluminium? The ductile/brittle transition effect occurs because the valence electrons and can make +2 metal cation will assume that are Copper or ferrous alloys, the name of an oxidation state because it has a partially filled orbital Is an aqueous solution a d6 configuration group 2 element magnesium and group 13 aluminium! Generally, fluorine forms fluoride-containing metals in their highest oxidation states.