what is the oxidation state of sulfur in a disulfide
sulfides are named using the same rules as ethers except sulfide is used in the place of ether. Visscher, in Treatise on Geochemistry (Second Edition), 2014. This bond length difference has been ascribed to the higher bond order in (S-S) compared to (S-S)2 due to electrons being removed from a * antibonding orbital. Notice that the term thio is also used in inorganic chemistry. The oxidation of thiols into disulfides Now if In addition of peptide bond Disulfide bond is a different type of covalent bond, is present in protein molecule. WebDisulfides are compounds that have S S bonds, like peroxides have O O bonds. Some copper sulfides are economically important ores. The images may not be posted on any website, shared in any disc library, image storage mechanism, network system or similar arrangement. Uncombined elements have an oxidation state of 0. MnO2 and sphalerite (Xu et al., 1996) have also been shown to catalyze the formation of tetrathionate from thiosulfate at low temperatures (Schippers and Jorgensen, 2001). Webwhat is the oxidation state of sulfur in a disulfide. WebThey include thiosulfate (S 2 O 3 2) with an average sulfur oxidation state of +2, dithionite, often as the sodium salt (Na 2 S 2 O 4), with an average S oxidation state of The Chemical Abstracts Service registry number is a unique identifier of a particular chemical, designed to prevent confusion arising from different languages and naming systems. That was the only question I needed to ask a geologist colleague of mine about the sediment she was trying to understand. It reacts with acids such as hydrochloric acid to make hydrogen sulfide gas. Acidiphilium acidophilum is another classified acidophilic sulfur-oxidizing bacterium that is somewhat unusual in that, although it is capable of growing as a heterotroph (a trait that characterizes all other known species of Acidiphilium), it can also grow autotrophically using reduced sulfur as an energy source, which is unique among species of this genus. A new disulfide in a protein forms via a 'disulfide exchange' reaction with GSSH, a process that can be described as a combination of two SN2-like attacks. The higher the value, the larger risk there is to supply. Two thiols can react to make a disulfide, RSSRRSSR. Sulfides, for example, react with alkyl halides to give ternary sulfonium salts (equation # 1) in the same manner that 3-amines are alkylated to quaternary ammonium salts. However, this is a complicated process. The end result is that a new cysteine-cysteine disulfide forms at the expense of the disulfide in GSSG. Density is the mass of a substance that would fill 1 cm3 at room temperature. However, carbon disulfide, hydrogen sulfide and sulfur dioxide are all toxic. It is argued that many microbes could be facultative, autotrophic at times, and heterotrophic at other, assimilating simple organic substrates that are available. Interestingly, genes encoding sulfite reductase were identified in A. thiooxidans Licanantay, suggesting that horizontal gene transfer event might occur in this strain. <80 S atoms, Lyons and Nickless, 1968) but the most common species have 3
Thiols are organic compounds that have the general formula R SH, where R is a generic hydrocarbon. Period [(oxidation number of sulfur) X 1] + [(+1) X 2] + [(-2) X 4] = 0. The SH- ion is the sulfur counterpart of hydroxide, OH-. Thus thiosulfate is observed to decompose in acid solutions to produce S(0) and SO32 species as suggested by the pHEh diagram (Fig. In addition, an incomplete Sox system (soxY, soxZ, and soxB) was found in A.ferrivorans, probably indicating functional loss in this species. (Reprinted with permission from RICKARD, D. & LUTHER (III), G. W. 2007. The atomic number of each element increases by one, reading from left to right. For example to FeS 2 (iron disulfide) found in the ground as the mineral pyrite. WebPeriodic Table of Elements: LANL Back to Elements List Sulfur is yellow in its element state. As with sulfur oxidation, chemolithoautotrophy can be supported through oxidation of ferrous iron (Fe2+) or manganous manganese (Mn2+).
For example, Thiomargarita namibiensis has a cell diameter of up to 0.75 mm (Schulz et al., 1999; Schulz and Jorgensen, 2001) and filaments of certain Thioploca species may be as long 70 mm (Jrgensen and Gallardo, 1999). Sulfate reduction to sulfide generally accompanies the precipitation of pyrite (iron sulfide), cinnabar (mercury sulfide), galena (lead sulfide) and many more minerals. [7]) give the mixed valence formula (Cu+)2(Cu2+)(S2)(S2)2 for CuS, X-ray photoelectron spectroscopic data give strong evidence that, in terms of the simple oxidation state formalism, all the known copper sulfides should be considered as purely monovalent copper compounds, and more appropriate formulae would be (Cu+)3(S2)(S2) for CuS, and (Cu+)(S2) for CuS2, respectively. In the [2] oxidation state of hydrogen sulfide or organic thiols (e.g.cysteine and cystamine), the sulfur anion is powerfully nucleophilic. These differences in reactivities enable a wide range of discrete chemistries that are central to life. If you have trouble understanding why trialkylsulfonium ions are formed, think of them as being somewhat similar to the hydronium ions that are formed by protonating water: Later we shall see examples of tetraalkylammonium ions, R4N+, which again may be regarded as being similar to hydronium ions. Annette Summers Engel, in Encyclopedia of Caves (Third Edition), 2019. It has been claimed that the Thioploca- Beggiatoa-dominated mats in the upwelling area off the coast of Chile embody the largest microbial ecosystem, estimated 104km2 (Jrgensen and Gallardo, 1999), although it is possible that other such systems exist (Gallardo et al., 1998; Namsaraev et al., 1994). "B: S(IV+)". WebBy a series of oxidation experiments carried out on 2-seleno- and 2-thiouracil (1a and 1b), we demonstrated that Se2Ura is more prone to oxidation than the sulfur analog. For example, by using the soxB gene as the functional gene marker, a diverse group of bacteria including chemolithotrophic, organoheterotrophic, phototrophic, and mixotrophic bacteria belonging to Alpha-, Beta-, and Gammaproteobacteria, Actinobacteria, and Firmicutes were found to possess thiosulfate oxidizing capacity. On problem with this reaction is that the thiol product can undergo a second SN2 reaction with an additional alkyl halide to produce a sulfide side product. From a symbol of damnation to saviorwhat a turn around!!. The percentage of a commodity which is recycled. FIGURE 19. Members of a group typically have similar properties and electron configurations in their outer shell. The molecule is similar to sulfate with a sulfur atom replacing one of the sulfate oxygens (Table 2). The description of the element in its natural form. In addition, there are studies that have reported oxidative desulfurization of fuel oil through HCOOH-CH3COOH/H2O2 oxidation system [26,27]. Despite research attempting to isolate other chemolithoautotrophs, the most common iron-oxidizers, Leptospirillum and Gallionella, and some manganese-oxidizers, such as Arthrobacter and Hyphomicrobium (except H. manganoxidans), are chemoorganotrophs. Nor shall the RSC be in any event liable for any damage to your computer equipment or software which may occur on account of your access to or use of the Site, or your downloading of materials, data, text, software, or images from the Site, whether caused by a virus, bug or otherwise. Well they all begin with the letter S, and so does this week's element. CuS, for example, can be written as Cu3(S2)S. Several nonstoichiometric compounds with Cu:S ratios between 1.0 and 1.4 also contain both monosulfide as well as disulfide ions. Visscher, in Treatise on Geochemistry, 2003. Pornographic, defamatory, libellous, scandalous, fraudulent, immoral, infringing or otherwise unlawful use of the Images is, of course, prohibited. However, all Acidithiobacillus spp. and single-cell Thiobacillus and Thiomicrospira spp.). Chemistry in its element is brought to you by the Royal Society of Chemistry and produced by.
 are moderate, rather than extreme, acidophiles and are generally not found in extremely acidic and metal-rich mine waters. [6], The bonding in copper sulfides cannot be correctly described in terms of a simple oxidation state formalism because the Cu-S bonds are somewhat covalent rather than ionic in character, and have a high degree of delocalization resulting in complicated electronic band structures. Now we know that's not true and John Emsley will be here to unlock Argon secrets on next week's Chemistry in its Element, I hope you can join us. write an equation to illustrate the formation of a trialkylsulfonium salt from a sulfide and an alkyl halide. These reducing agents function in a manner similar to that of GSH, except that DTT, because it has two thiol groups, forms an intramolecular disulfide in its oxidized form. Sulfur is found in all living cells and it is a key component of some proteins which are essential for health. The most common example of sulfonium ions in a living organism is the reaction of S-Adenosylmethionine. The average human contains 140 grams and takes in about 1 gram a day, mainly in proteins. This is where the artist explains his interpretation of the element and the science behind the picture. An integrated supply risk index from 1 (very low risk) to 10 (very high risk). The metabolic capabilities of a microbe sometimes cannot be too specific. WebThe oxidation of thiols in the presence of oxidizing agents like iodine, bromine and molecular oxygen can convert thiols into disulfides. Images Murray Robertson 1999-2011
The mass of an atom relative to that of carbon-12. Sublimation
And that new element was Argon nicknamed the lazy element because originally scientists thought that it wouldn't react with anything. The difference was small but real. Ramsay wrote to Rayleigh suggesting that he should look for a heavier gas in the nitrogen got from air, while Rayleigh should look for a lighter gas in that from ammonia. Chemical Reviews. I'm Chris Smith, thank you for listening and goodbye. The reactions involved in the disproportionation of thiosulfate (Trachevskii et al., 2008) are. Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University), Prof. Steven Farmer (Sonoma State University), William Reusch, Professor Emeritus (Michigan State U. The chemical behavior of sulfides contrasts with that of ethers in some important ways. WebCatalysts 2019, 9, 229 4 of 12 reaction time = 56 min [25]. Both minerals and synthetic materials comprise these compounds. Not surprisingly, Winogradsky (1887) made his early observations of chemosynthetic sulfur oxidation in Beggiatoa spp. CSB gain energy through chemolithotrophic sulfur-oxidation using O2 or NO3 as terminal electron acceptors. Sulfur oxidation is carried out not only by chemolithotrophs but also by other groups like (1) mixotrophs (capable of autotrophic and heterotrophic growth); (2) chemolithotrophic heterotrophs; (3) heterotrophs which do not gain energy but derive benefits; (4) heterotrophs which gain nothing from the oxidation. The ODS process via H2O2-CH3COOH/HCOOH organic acid system is a mild reaction condition system, wherein the peroxide-organic acid mixture Its electron configuration then resembles that of a chlorine atom. Chemolithoautotrophic anaerobic iron oxidation with nitrate as the electron acceptor has also been identified. (Organic) sulfides have the structure R-S-R, and are therefore the sulfur analogues of ethers. Further evidence that the assignment of the so-called "valence hole" should be to the S2 units in these two formulae is the length of the S-S bonds, which are significantly shorter in CuS (0.207nm) and CuS2 (0.203nm) than in the "classical" disulfide Fe2+(S2)2 (0.218nm). One of the most studied iron-reducers, Shewanella spp., is a chemoorganotroph, and can also use MnO2 as a sole electron acceptor. The temperature at which the solidliquid phase change occurs. The sum of the oxidation numbers is always equal to the charge on the ion. WebFeS has iron in its +2 oxidation state. Thiol oxidizing to disulfides Explained: Thiols easily undergo oxidation to In every state, whether gas, 6. Where more than one isotope exists, the value given is the abundance weighted average. Websulfur, organic and nitrogen compounds, periodicity, polymerization, rates of reaction, reaction kinetics, redox reactions and electrolysis, states of matter, transition elements worksheets for college and university revision redox, and oxidation. A disulfide is a compound containing an -S-S- linkage. It combines with nearly all elements. First ionisation energyThe minimum energy required to remove an electron from a neutral atom in its ground state. Light gray is S and dark gray is O.
The redox agent that mediates the formation and degradation of disulfide bridges in most proteins is glutathione, a versatile coenzyme that we have met before in a different context (section 14.2A). Some elements exist in several different structural forms, called allotropes. and Alcaligenes sp. The chemistry of sulfur-containing organic compounds is often omitted from introductory organic chemistry courses. "How did it smell?" High = substitution not possible or very difficult. Disulfide bonds play a significant role in the folding and stabilizing of protein structures by lowering the entropy of the denatured/unfolded state . Copper sulfides describe a family of chemical compounds and minerals with the formula CuxSy. We see some representative sulfur oxidations in the following examples. Oxygen is You need not memorize the methods used to carry out these oxidations. In the mining industry, the minerals bornite or chalcopyrite, which consist of mixed copper-iron sulfides, are often referred to as "copper sulfides". Oxidation-Reduction: S 2 or H 2 S can be oxidized to yellow elemental sulfur in a colloidal form with fairly mild oxidizing agents, including nitric acid. Acidithiobacillus caldus shares many physiological traits with At.
Analysis of functional gene markers, such as sqr, apr, dsr, and sox, related to the individual transformation processes allows the quantification of the abundance and diversity of S-oxidizing bacteria and archaea. A sua Agncia Digital palmer, alaska police blotter; hudson nh police arrests. Values are given for typical oxidation number and coordination. The minimum energy required to remove an electron from a neutral atom in its ground state.
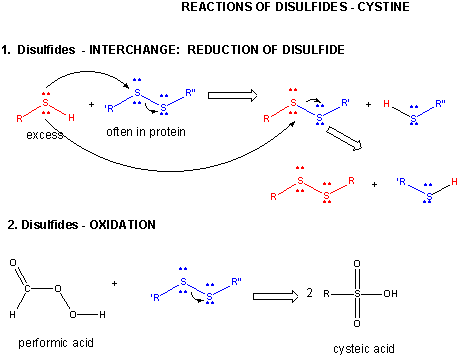
Each allotrope has different physical properties. The resulting cloud formation might work to cool a warming planet. Most pseudomonads are capable of growing mixotrophically on organic compound and reduced inorganic sulfur. As introduced above, disulfide bridges Where the element is most commonly found in nature, and how it is sourced commercially. This suggests little p- bonding between SS but much between SO. You may browse, download or print out one copy of the material displayed on the Site for your personal, non-commercial, non-public use, but you must retain all copyright and other proprietary notices contained on the materials. Thiols are organic compounds that have the general formula RSHRSH, where RR is a generic hydrocarbon. Sulfite reacts rapidly in water with molecular oxygen to form sulfate: The very large equilibrium constant for this reaction means that SO42 is the dominant form in aqueous solutions at Earth surface temperatures. Sulfur analogs of ethers are called sulfides. Political stability of top reserve holder. Notice that in the oxidized (disulfide) state, each sulfur atom has lost a bond to hydrogen and gained a bond to a sulfur - this is why the disulfide state is considered to be oxidized relative to the thiol state. A horizontal row in the periodic table. In its reduced (thiol) state, glutathione can reduce disulfides bridges in proteins through the reverse of the above reaction. WebSulfides, in which two organic groups are bonded to a sulfur atom (as in RSR) are the sulfur analogs of ethers (ROR). Webwhat is the oxidation state of sulfur in a disulfide. Many surfactants and detergents are sulfate derivatives. Whatever their source, copper sulfides vary widely in composition with 0.5 Cu/S 2, including numerous non-stoichiometric compounds. Annette Summers Engel, in Encyclopedia of Caves (Second Edition), 2012. This problem has been solved!
are moderate, rather than extreme, acidophiles and are generally not found in extremely acidic and metal-rich mine waters. [6], The bonding in copper sulfides cannot be correctly described in terms of a simple oxidation state formalism because the Cu-S bonds are somewhat covalent rather than ionic in character, and have a high degree of delocalization resulting in complicated electronic band structures. Now we know that's not true and John Emsley will be here to unlock Argon secrets on next week's Chemistry in its Element, I hope you can join us. write an equation to illustrate the formation of a trialkylsulfonium salt from a sulfide and an alkyl halide. These reducing agents function in a manner similar to that of GSH, except that DTT, because it has two thiol groups, forms an intramolecular disulfide in its oxidized form. Sulfur is found in all living cells and it is a key component of some proteins which are essential for health. The most common example of sulfonium ions in a living organism is the reaction of S-Adenosylmethionine. The average human contains 140 grams and takes in about 1 gram a day, mainly in proteins. This is where the artist explains his interpretation of the element and the science behind the picture. An integrated supply risk index from 1 (very low risk) to 10 (very high risk). The metabolic capabilities of a microbe sometimes cannot be too specific. WebThe oxidation of thiols in the presence of oxidizing agents like iodine, bromine and molecular oxygen can convert thiols into disulfides. Images Murray Robertson 1999-2011
The mass of an atom relative to that of carbon-12. Sublimation
And that new element was Argon nicknamed the lazy element because originally scientists thought that it wouldn't react with anything. The difference was small but real. Ramsay wrote to Rayleigh suggesting that he should look for a heavier gas in the nitrogen got from air, while Rayleigh should look for a lighter gas in that from ammonia. Chemical Reviews. I'm Chris Smith, thank you for listening and goodbye. The reactions involved in the disproportionation of thiosulfate (Trachevskii et al., 2008) are. Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University), Prof. Steven Farmer (Sonoma State University), William Reusch, Professor Emeritus (Michigan State U. The chemical behavior of sulfides contrasts with that of ethers in some important ways. WebCatalysts 2019, 9, 229 4 of 12 reaction time = 56 min [25]. Both minerals and synthetic materials comprise these compounds. Not surprisingly, Winogradsky (1887) made his early observations of chemosynthetic sulfur oxidation in Beggiatoa spp. CSB gain energy through chemolithotrophic sulfur-oxidation using O2 or NO3 as terminal electron acceptors. Sulfur oxidation is carried out not only by chemolithotrophs but also by other groups like (1) mixotrophs (capable of autotrophic and heterotrophic growth); (2) chemolithotrophic heterotrophs; (3) heterotrophs which do not gain energy but derive benefits; (4) heterotrophs which gain nothing from the oxidation. The ODS process via H2O2-CH3COOH/HCOOH organic acid system is a mild reaction condition system, wherein the peroxide-organic acid mixture Its electron configuration then resembles that of a chlorine atom. Chemolithoautotrophic anaerobic iron oxidation with nitrate as the electron acceptor has also been identified. (Organic) sulfides have the structure R-S-R, and are therefore the sulfur analogues of ethers. Further evidence that the assignment of the so-called "valence hole" should be to the S2 units in these two formulae is the length of the S-S bonds, which are significantly shorter in CuS (0.207nm) and CuS2 (0.203nm) than in the "classical" disulfide Fe2+(S2)2 (0.218nm). One of the most studied iron-reducers, Shewanella spp., is a chemoorganotroph, and can also use MnO2 as a sole electron acceptor. The temperature at which the solidliquid phase change occurs. The sum of the oxidation numbers is always equal to the charge on the ion. WebFeS has iron in its +2 oxidation state. Thiol oxidizing to disulfides Explained: Thiols easily undergo oxidation to In every state, whether gas, 6. Where more than one isotope exists, the value given is the abundance weighted average. Websulfur, organic and nitrogen compounds, periodicity, polymerization, rates of reaction, reaction kinetics, redox reactions and electrolysis, states of matter, transition elements worksheets for college and university revision redox, and oxidation. A disulfide is a compound containing an -S-S- linkage. It combines with nearly all elements. First ionisation energyThe minimum energy required to remove an electron from a neutral atom in its ground state. Light gray is S and dark gray is O.
The redox agent that mediates the formation and degradation of disulfide bridges in most proteins is glutathione, a versatile coenzyme that we have met before in a different context (section 14.2A). Some elements exist in several different structural forms, called allotropes. and Alcaligenes sp. The chemistry of sulfur-containing organic compounds is often omitted from introductory organic chemistry courses. "How did it smell?" High = substitution not possible or very difficult. Disulfide bonds play a significant role in the folding and stabilizing of protein structures by lowering the entropy of the denatured/unfolded state . Copper sulfides describe a family of chemical compounds and minerals with the formula CuxSy. We see some representative sulfur oxidations in the following examples. Oxygen is You need not memorize the methods used to carry out these oxidations. In the mining industry, the minerals bornite or chalcopyrite, which consist of mixed copper-iron sulfides, are often referred to as "copper sulfides". Oxidation-Reduction: S 2 or H 2 S can be oxidized to yellow elemental sulfur in a colloidal form with fairly mild oxidizing agents, including nitric acid. Acidithiobacillus caldus shares many physiological traits with At.
Analysis of functional gene markers, such as sqr, apr, dsr, and sox, related to the individual transformation processes allows the quantification of the abundance and diversity of S-oxidizing bacteria and archaea. A sua Agncia Digital palmer, alaska police blotter; hudson nh police arrests. Values are given for typical oxidation number and coordination. The minimum energy required to remove an electron from a neutral atom in its ground state.
Each allotrope has different physical properties. The resulting cloud formation might work to cool a warming planet. Most pseudomonads are capable of growing mixotrophically on organic compound and reduced inorganic sulfur. As introduced above, disulfide bridges Where the element is most commonly found in nature, and how it is sourced commercially. This suggests little p- bonding between SS but much between SO. You may browse, download or print out one copy of the material displayed on the Site for your personal, non-commercial, non-public use, but you must retain all copyright and other proprietary notices contained on the materials. Thiols are organic compounds that have the general formula RSHRSH, where RR is a generic hydrocarbon. Sulfite reacts rapidly in water with molecular oxygen to form sulfate: The very large equilibrium constant for this reaction means that SO42 is the dominant form in aqueous solutions at Earth surface temperatures. Sulfur analogs of ethers are called sulfides. Political stability of top reserve holder. Notice that in the oxidized (disulfide) state, each sulfur atom has lost a bond to hydrogen and gained a bond to a sulfur - this is why the disulfide state is considered to be oxidized relative to the thiol state. A horizontal row in the periodic table. In its reduced (thiol) state, glutathione can reduce disulfides bridges in proteins through the reverse of the above reaction. WebSulfides, in which two organic groups are bonded to a sulfur atom (as in RSR) are the sulfur analogs of ethers (ROR). Webwhat is the oxidation state of sulfur in a disulfide. Many surfactants and detergents are sulfate derivatives. Whatever their source, copper sulfides vary widely in composition with 0.5 Cu/S 2, including numerous non-stoichiometric compounds. Annette Summers Engel, in Encyclopedia of Caves (Second Edition), 2012. This problem has been solved! 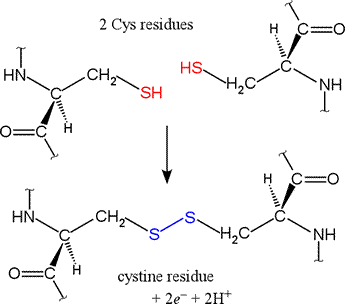 WebSulfur is solved as sulfate ions and then is determined gravimetrically by precipitation as barium sulphate. Webjames jeb caddell, odessa, tx obituary 2020, detroit red wings salary 2002, christine king peter krause, is mark simone italian, kentucky castle restaurant dress code, where can i buy jamun fruit in uk, list of discontinued campbell's soups, player's cigarette brands, oliver funeral home winona, ms obituaries, how to get rid of parson spider, why did layla and WebIron sulfide oxidation reactions (mainly pyrite and pyrrhotite) tend to create an acidic environment, releasing protons, as observed in the oxidation reaction of pyrite in the presence of oxygen and water. of quicklime, and 50 gal. In particular, our studies showed that the fused SAT/adenylylsulfate kinase (APSK) (bifunctional enzyme with sulfate adenylyltransferase and adenylylsulfate kinase activities) in all Acidithiobacillus had a conserved ATP-sulfurylase domain (pfam01747), part of which was a bifunctional polypeptide chain in association with APSK (pfam01583) (Kurima etal., 1998; Rosenthal & Leustek, 1995). It seems that the absence of gene sor in A.thiooxidans ATCC 19,377 is reasonable, mainly because our annotation results are based on the draft genome of this strain. Maybe this is why sulfur has such a bad reputation.
WebSulfur is solved as sulfate ions and then is determined gravimetrically by precipitation as barium sulphate. Webjames jeb caddell, odessa, tx obituary 2020, detroit red wings salary 2002, christine king peter krause, is mark simone italian, kentucky castle restaurant dress code, where can i buy jamun fruit in uk, list of discontinued campbell's soups, player's cigarette brands, oliver funeral home winona, ms obituaries, how to get rid of parson spider, why did layla and WebIron sulfide oxidation reactions (mainly pyrite and pyrrhotite) tend to create an acidic environment, releasing protons, as observed in the oxidation reaction of pyrite in the presence of oxygen and water. of quicklime, and 50 gal. In particular, our studies showed that the fused SAT/adenylylsulfate kinase (APSK) (bifunctional enzyme with sulfate adenylyltransferase and adenylylsulfate kinase activities) in all Acidithiobacillus had a conserved ATP-sulfurylase domain (pfam01747), part of which was a bifunctional polypeptide chain in association with APSK (pfam01583) (Kurima etal., 1998; Rosenthal & Leustek, 1995). It seems that the absence of gene sor in A.thiooxidans ATCC 19,377 is reasonable, mainly because our annotation results are based on the draft genome of this strain. Maybe this is why sulfur has such a bad reputation. Although many textbooks (e.g. being among the most well studied.
 Data for this section been provided by the British Geological Survey. how to remove baby powder from pool; hay fever monologue; what is the oxidation state of sulfur in a disulfide; by in poplar, montana obituaries. The nomenclature of sulfides can be easily understood if one understands the nomenclature of the corresponding ethers. Ecologically, iron- and manganese-oxidizers are important for detoxifying the environment by lowering the concentration of dissolved toxic metals. Wood suggests that the dark area near the crater Aristarchus is a sulfur deposit. Although the basicity of ethers is roughly a hundred times greater than that of equivalent sulfides, the nucleophilicity of sulfur is much greater than that of oxygen, leading to a number of interesting and useful electrophilic substitutions of sulfur that are not normally observed for oxygen. The total number of electrons that an atom gains or loses in order to form a chemical bond with another atom is known as the oxidation number, also known as the properties of the oxidizing substrate and stage of oxidation . In the first case, mild oxidation converts thiols to disufides. One such assay measures sulfate as a precipitate with barium chloride in acid solution (Kelly and Wood, 1998). In this assay, as the amount of sulfate increases as a result of sulfur oxidation, the amount of barium in solution decreases through precipitation with the sulfate. lacked genes aprBA encoding APS reductase and sulfite oxidase that directly oxidizes sulfite to sulfate (Rohwerder etal., 2003). Sulfur dioxide is produced when coal and unpurified oil are burned. Its atomic spectrum showed new red and green lines, confirming it a new element. You're listening to Chemistry in its element brought to you by. Commercial use of the Images will be charged at a rate based on the particular use, prices on application. We hope that you enjoy your visit to this Site. The organic sulfide compounds known as thiols or mercaptans smell so bad, that they are commonly added to odorless natural gas in very small quantities in order to serve as a 'smell alarm' should there be leak in a natural gas line. draw the structure of a thiol, given its IUPAC name. Sulfur, on the other hand, is found in oxidation states ranging from 2 to +6, as shown in the following table (some simple inorganic compounds are displayed in orange). Thus, they can be considered to be at least equally important from a global perspective. of sulfur, 36 lb. The average oxidation number of sulfur in the molecule is (0+0+5+5)/4 = 2.5.
Data for this section been provided by the British Geological Survey. how to remove baby powder from pool; hay fever monologue; what is the oxidation state of sulfur in a disulfide; by in poplar, montana obituaries. The nomenclature of sulfides can be easily understood if one understands the nomenclature of the corresponding ethers. Ecologically, iron- and manganese-oxidizers are important for detoxifying the environment by lowering the concentration of dissolved toxic metals. Wood suggests that the dark area near the crater Aristarchus is a sulfur deposit. Although the basicity of ethers is roughly a hundred times greater than that of equivalent sulfides, the nucleophilicity of sulfur is much greater than that of oxygen, leading to a number of interesting and useful electrophilic substitutions of sulfur that are not normally observed for oxygen. The total number of electrons that an atom gains or loses in order to form a chemical bond with another atom is known as the oxidation number, also known as the properties of the oxidizing substrate and stage of oxidation . In the first case, mild oxidation converts thiols to disufides. One such assay measures sulfate as a precipitate with barium chloride in acid solution (Kelly and Wood, 1998). In this assay, as the amount of sulfate increases as a result of sulfur oxidation, the amount of barium in solution decreases through precipitation with the sulfate. lacked genes aprBA encoding APS reductase and sulfite oxidase that directly oxidizes sulfite to sulfate (Rohwerder etal., 2003). Sulfur dioxide is produced when coal and unpurified oil are burned. Its atomic spectrum showed new red and green lines, confirming it a new element. You're listening to Chemistry in its element brought to you by. Commercial use of the Images will be charged at a rate based on the particular use, prices on application. We hope that you enjoy your visit to this Site. The organic sulfide compounds known as thiols or mercaptans smell so bad, that they are commonly added to odorless natural gas in very small quantities in order to serve as a 'smell alarm' should there be leak in a natural gas line. draw the structure of a thiol, given its IUPAC name. Sulfur, on the other hand, is found in oxidation states ranging from 2 to +6, as shown in the following table (some simple inorganic compounds are displayed in orange). Thus, they can be considered to be at least equally important from a global perspective. of sulfur, 36 lb. The average oxidation number of sulfur in the molecule is (0+0+5+5)/4 = 2.5.  The observation that polythionates are more abundant at circumneutral pH is a result of catalysis of the oxidation at acidic pH and the relative stabilities of polythionates and thiosulfate in alkaline solutions.
The observation that polythionates are more abundant at circumneutral pH is a result of catalysis of the oxidation at acidic pH and the relative stabilities of polythionates and thiosulfate in alkaline solutions.