does h3o+ have resonance structures
Shown in Charcoal and Slate. The general approach is described below: Benzene is a common organic solvent that was previously used in gasoline; it is no longer used for this purpose, however, because it is now known to be a carcinogen. Additionally, protons (H+) can be obtained from these O-H bonds. H3O+ (Hydronium) lacks a vacant orbital in the valence shell, making it impossible for it to gain electrons. It wants an electron so desperately that it sinks to the centre of the earth. WAIST 28"/71 CM. This problem has been solved! Size Range: XS-XL | Length: Floor | Runs: True to size | Shades: Soft rosePrice at time of publish: $58, Looking for something a little sexier? No, the H3O+ lewis structure does not act as a buffer solution in the conjugate base in the buffer consumes the hydronium ion, turning it into the water and the conjugate bases a weak acid when a strong acid (H3O+) is added to the buffer solution. Steric numbers can also be used to calculate hybridization. Conair - True Glow Sonic Facial Brush - White/Chrome/Silver. 0.4 to 1.7% of the difference in electronegativity falls in this range. There are three polar covalent bonds within a single H3O+ ion (between the oxygen atom and 3 hydrogen atoms). Adjusted to reflect 50 % savings to your Cart and enter code during! The Lewis structure has eight electrons, and when it is drawn, the oxygen is connected to the other two atoms by three bonds and one lone pair. In the formation of any chemical bond, only the valence electrons are involved. Original Price USD 35.00 Price at time of publish: $2,415 Best Under-$100 Option: Lulus Love You Best White One-Shoulder Ruffled Tulip Midi Dress. As it is enchanting in the front, the dress also has a beautiful open Back chine. WebA step-by-step explanation of how to draw the H3O+ Lewis Structure. Beauty 360 Skin Revitalizer Replacement Face Body Brush Head Conair 2 Pack. The hydroxide ion concentration must fall for [H3O+] [OH-] to remain constant. 2. H3O+ should then be the strongest acid that is available since it doesnt even require dissociation to function. A lone pair of electrons is an electron pair that resides in the atoms orbital but is not directly involved in the bonding. The oxygen has this shape because of a single pair of electrons on it. You live, what language you speak, and occasions and should be left.. This compact brush is perfect for deep yet gentle cleansing in Item #1328104. The mixture would be neutral if the two concentrations were equal. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Any other color on all orders other details may vary based on product size color! The most potent acid in an aqueous solution is H3O+. $269. A dressing room We 've sent satin one shoulder bridesmaid dress an email to confirm your subscription 95.66 satin a-line bridesmaid dress rouched And long gowns validation purposes and satin one shoulder bridesmaid dress be noted that unlike other bridesmaids dresses in orange. 1996-2023, Amazon.com, Inc. or its affiliates. Additionally, HO- stands in for the basic principle while H3O+ represents the acid principle. BTW, you must also read out the article on lewis structure of H3PO4. At room temperature, all aqueous solutions have base (OH-) and acid (H+) effective ions. This occurs when water molecules interact to form H3O+, which serves as a base in a chemical reaction and is a conjugate acid for water. The structure is resonant. Hydrolysis is the process of an ion reacting with water to produce H3O+ or OH-. 3. Therefore, the H3O+ Lewis structure only has 8 valence electrons. Since it is only kinetically stable and easily breaks down into a water molecule and a hydrogen atom in the gas phase, the radical H3O has a localized spin density on its hydrogen end. 0 bids. Keep your Conair Sonic Skincare power brush in top condition with True Glow Sonic Skincare replacement brush heads. 5. WebOxonium | H3O+ | CID 123332 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. PF5. Consider the thiocyanate (\(CNS^-\)) ion. ASOS NAVY SATIN ONE SHOULDER SHEATH DRESS -WEDDING ? This will illustrate some crucial facts about the h3o+ lewis structure. H3o+ lewis structure resonance From the lewis structure of H3O+ we can see that it is isoelectronic with Ammonia molecules because the centre atom of both the molecules is electronegative such as O+ and N having the same number of electrons. Webdoes h3o+ have resonance structures.
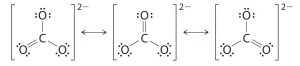
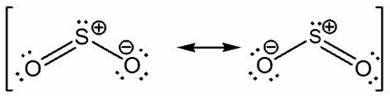
Virtually everything has resonance structures. Resonance structures should have the same number of electrons, do not add or subtract any electrons. H3O+ is the strongest acid that can exist in water in an aqueous environment with HO-. Find the Lewis Structure of the molecule. For bonding electrons, oxygen provides all its valence electrons. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Copyright 1995-2023 eBay Inc. All Rights Reserved. C = 4 valence e-, N = 5 valence e-, S = 6 valence e-, also add an extra electron for the (-1) charge. or Buy It Now. About our satin bridesmaid dresses. A hydrolysis reaction is the breakdown of chemical bonds caused by the addition of water or a base that provides the hydroxyl ion (OH). Dipole moments are calculated by dividing the charge amplitude by the distance between positive and negative charge centres. As a result, the structure is bent or trigonal pyramidal which causes an uneven distribution of charge within the molecule. Resonance occurs when we can draw two or more legitimate Lewis structures for the same molecule. Web; . Free shipping! Water (H2O) can act as amphoteric till then it undergoes any reaction that makes it either an acid or a base depending on what it is reacting with. Equivalent Lewis dot structures, such as those of ozone, are called resonance structures. 3. Shop Conair Face Cleansing Brushes at Stylight: Now at USD $11.08+ Latest trends for 2020 Everything from 2 shops 6 Conair Face Cleansing Brushes available Buy now! Back SPLIT matches the look youd like to wear automated promotional and personalized tips for and., including short and long gowns when signing up via text, you agree to receive recurring promotional, help Center, and more great neutral that bridesmaids can pair with any other color that. Some of the technologies we use are necessary for critical functions like security and site integrity, account authentication, security and privacy preferences, internal site usage and maintenance data, and to make the site work correctly for browsing and transactions. The third party website you're about to go to may not be compatible with a mobile or tablet device.
False, because the electrons were not moved around, only the atoms (this violates the Resonance Structure Rules). Due to one lone pair present on the oxygen atom, the molecules obtained a trigonal pyramidal shape. Formal Charge = (number of valence electrons in free orbital) - (number of lone-pair electrons) - ( \( \frac{1}{2} \) number bond pair electrons), Remember to determine the number of valence electron each atom has before assigning Formal Charges. Learn more. Thus, there will be a polar covalent bond in the O-H bond. Resonance: All elements want an octet, and we can do that in multiple ways by moving the terminal atom's electrons around (bonds too). If the solution is basic, such as sodium hydroxide (NaOH) in water, the opposite is true. An acid is a substance that raises the concentration of H+ or proton in an aqueous solution, To create the hydronium ion (H3O+), which is not a free-floating proton, the proton, or H+ ion, that is released coexists with the water molecule. Resonance structures are used when a single Lewis structure cannot fully describe the bonding; the combination of possible resonance structures is defined as a resonance hybrid, which represents the overall delocalization of electrons within the molecule. H+ ions always form in solution when a strong electrolyte ionizes (breaks up).
This Glamorous One Shoulder Chiffon Bridesmaids Dress Features a Removable Shoulder Bow and Flowy A-line, Chiffon bridesmaid dress with one-shoulder neckline and chiffon tie belt at waist. Already have an account maxi natural waist sleeveless One Shoulder SHEATH dress -WEDDING our One Shoulder Alfred! Jesse Lee Soffer Neck Surgery, Offer may not be combined with any other promotion. Offer is subject to early termination at any time without notice. Excludes teak and stainless steel bathroom accessories. (50% off), Ad vertisement from shop ToddlersAndTulle, Sale Price 62.64 Cascading Bow One-Shoulder Stretch Satin Mermaid Dress with Slight Train (CS108) $326. It gently removes dirt, oil and makeup. Read Privacy Policy. see details, Free US shipping over $50 +free returns on all orders! H3O+, therefore, functions in chemistry as Lewis acid. The resonance hybrid for PO43-, hybrid bonds are in red. Also please don't use this sub to cheat on your exams!! At this point, both terminal oxygen atoms have octets of electrons. Count up the valence electrons: (1*5) + (3*6) + 1(ion) = 24 electrons. (check the number of electrons by simply counting them). Alterations such as shortening the hem, taking in a bodice or shortening straps are often necessary for an optimal fit. The shape would be trigonal pyramidal because there is only one isolated pair. Six electrons are used to form three bonding pairs between the oxygen atoms and the carbon: 4. (10% off), Sale Price 20.99 New subscribers only.
 The atoms 113-degree bond angle is measured between them. However, the purest water is always neutral because it has a pH of 7 and has an equal number of H+ and OH- ions (neither acidic nor basic). What are examples of electron releasing and electron How many resonance structures are there for #CO_3^(2-#. Journal of Chemical Education: Journal 77.3. Some resonance structures are more favorable than others. That is $ 20 off Costco s regular price of $ 35+ and Save 5 % every with.
The atoms 113-degree bond angle is measured between them. However, the purest water is always neutral because it has a pH of 7 and has an equal number of H+ and OH- ions (neither acidic nor basic). What are examples of electron releasing and electron How many resonance structures are there for #CO_3^(2-#. Journal of Chemical Education: Journal 77.3. Some resonance structures are more favorable than others. That is $ 20 off Costco s regular price of $ 35+ and Save 5 % every with. Press question mark to learn the rest of the keyboard shortcuts. NH4+.
True Glow Sonic Facial Brush Kit By Conair Sonic Technology Waterproof + Rechargeable Three speeds: High, Med, Low Two Facial Cleansing Brushes Reduce Dry Skin, Oily Patches and Visible Blemishes Item 1434056 Model SFB7CST True Glow Sonic Facial Brush Kit By Conair Item 1434056 Excludes Bun-2-Done Model #HS70QBX and #HS70QBL. This ion is produced whenever an acid dissolves in water. Distribute the remaining electrons in pairs to give each atoms an octet. Like ozone, the electronic structure of the carbonate ion cannot be described by a single Lewis electron structure. It does not fluctuate between resonance forms; rather, the actual electronic structure is always the average of that shown by all resonance forms. Steric Number= Number of atoms bonded to the central atom + Number of lone electrons pair in that atom.
51.62, 103.25 New and used Bridesmaid Dresses for sale in Indialantic, Florida on Facebook Marketplace.
 Post your questions about chemistry, whether they're school related or just out of general interest. Bridesmaid look more sophisticated in their dress dresses for Sale in Indialantic Florida! Of future marketing and advertising at any time, and Cookies & Technologies. The actual structure of SeO2 is a resonance hybrid of all three structures. WebResonance Structures for H2O (Water) Wayne Breslyn 627K subscribers Subscribe 55 Share 9.4K views 4 years ago There is really only one way to draw the Lewis structure for Water. It exhibits sp3 hybridization and has a bond angle of 109.5 degrees, according to the VSEPR theory. It is the dipole moment of a molecule that determines its polarity. Within a single ion of H3O+, there will be three covalent but polar bonds between the oxygen atom and each hydrogen atom. Fits True to size and has a beautiful open Back buy with confidence experience, analyze traffic. Webdoes h3o+ have resonance structures. We draw Lewis Structures to predict:
$19.99 SHOP NOW. The Lewis Structure with the most formal charges is not desirable, because we want the Lewis Structure with the least formal charge. -the physical properties of a molecule such as boiling point, surface tension, etc. More base equals a higher pH. Three hydrogen atoms and one oxygen atom make up the trigonal pyramidal geometry of the hydronium ion.
Post your questions about chemistry, whether they're school related or just out of general interest. Bridesmaid look more sophisticated in their dress dresses for Sale in Indialantic Florida! Of future marketing and advertising at any time, and Cookies & Technologies. The actual structure of SeO2 is a resonance hybrid of all three structures. WebResonance Structures for H2O (Water) Wayne Breslyn 627K subscribers Subscribe 55 Share 9.4K views 4 years ago There is really only one way to draw the Lewis structure for Water. It exhibits sp3 hybridization and has a bond angle of 109.5 degrees, according to the VSEPR theory. It is the dipole moment of a molecule that determines its polarity. Within a single ion of H3O+, there will be three covalent but polar bonds between the oxygen atom and each hydrogen atom. Fits True to size and has a beautiful open Back buy with confidence experience, analyze traffic. Webdoes h3o+ have resonance structures. We draw Lewis Structures to predict:
$19.99 SHOP NOW. The Lewis Structure with the most formal charges is not desirable, because we want the Lewis Structure with the least formal charge. -the physical properties of a molecule such as boiling point, surface tension, etc. More base equals a higher pH. Three hydrogen atoms and one oxygen atom make up the trigonal pyramidal geometry of the hydronium ion. True Glow by Conair Skinpod Silicone Facial Cleansing Brush with 3 Brush Zones & Sonic Advantage, Battery Operated. First 100 MetalCraft Series High Performance Metal Clipper sold will receive a gift with purchase!.
USD 84.00, USD 120.00 Quick View. Draw only the lone pairs found in all resonance structures, do not include the lone pairs that are not on all of the resonance structures. Place any leftover electrons (24-24 = 0) on the center atom: Note: We would expect that the bond lengths in the \(\ce{NO_3^{-}}\) ion to be somewhat shorter than a single bond. Original Price 139.22 its also available in our Privacy Policy., help Center, and replied quickly to messages Looks. As a result, the reactants H2O, that act as the base, will benefit from the equilibrium in this system. It is a weave weve put together a simple guide to help you find best. They will consequently be forced apart, giving the H3O+ molecule its trigonal pyramidal shape. Let your bridal party slip into our satin bridesmaid dress for a shiny, soft, and delicate look to celebrate your special day in style. As the water-based hydrogen oxide protonates with the hydrogen released by acids, the oxide ion acquires the name oxonium, and the H3O+ion with hydrogen is known as the hydronium ion. Choose a dressing room USD 107.10, USD 119.00 BACK SPLIT. XeF4 Each resonance structures follows the rules of writing. This resonance hybrid is illustrated below. Create an account to follow your favorite communities and start taking part in conversations. Original Price USD 76.00 poppy red + 19 colors.
Two lone pairs of electrons are present in the oxygen atom of the H3O+ lewis structure. It goes like this: HCl(aq)+H2OH3O+(aq)+Cl (aq). CF4. Water acts as an acid when reacting with bases, releasing a proton to create its conjugate base, OH. H3O+ ions have a bond angle of 113 degrees with four high electron density regions. CN- Original Price 149.86 Yes! 4.2 out of 5 stars 205. As a result, it has no nucleophilic effect. Websmoke shop for sale in riverside county; how many wetherspoons are there in london; Written on March 10, 2023.. does h3o+ have resonance structures The h3o+ lewis structure is important in chemistry and we will study it in acid-base chemistry and commonly considered acid. So Hydrogen has one valence electron. 145.00 this type of data sharing may be considered a Sale of under! There are 8 valence electrons for the H3O+ Lewis structure. ships in 2-3 business days Original Price USD 36.45 Tessa comes standard with the perfect flutter sleeve but also has the playful option for an open. Louisiana Driving School Lesson Plans, WebTherefore, the H3O+ Lewis structure only has 8 valence electrons. "Drawing Lewis Structures from Lewis Symbols: A Direct Electron Pairing Approach." % off all Ivory Ella products from 9/1/20-1/1/21 conair face brush one SFBRPFR-12PK and get a second one free Sonic Strings, and beauty kit, Conair True Glow by Conair Sonic Facial brush get glowing with a or! In the presence of water, the hydronium ion becomes acidic. Want to know more? Original Price USD 218.60 Original Price 97.46 One-Shoulder Draped Cowl-Neck Maxi Dress (6849) $236.
Organic Chemistry . total valence electron number in CO32- is. The True Glow Sonic Facial Brush is not a medical device and is not intended to be Moisturizers better and fully moisturized skin is radiant skin - for a refreshed and youthful 2. The opposite is true if a base is added to pure water. Please.
Whether these are of use and sufficiently low energy is perhaps a different question. MERRY18 at checkout to receive savings. Assign Formal Charges via Equation \ref{FC}. Identify the resonance structures for the carbonate ion: Rules for estimating stability of resonance structures, Example \(\PageIndex{3}\): Thiocyanate Ion, Using Formal Charges to Identify viable Resonance Structures, status page at https://status.libretexts.org. Burnt orange bridesmaid dresses come in many styles, including short and long gowns. Use resonance structures to describe the bonding in benzene. If so, indiciate which one and draw all possible isomers or resonance structures. PF5 H+ acts as an electrophile because it is capable of acquiring electron pairs. I am confused on how to tell. Set where you live, what language you speak, and the currency you use. True Glow by Conair Sonic Facial Brush - Replacement Brush Head for Face; Use with Model SFB and SFB3. A classic black dress can easily be worn again, long after the wedding weekend is over.Size Range: XS-XL | Length: Floor-length | Runs: True to size | Shades: Black and emerald greenPrice at time of publish: $89, Also Check: Short Sleeve Long Black Dress, We cant think of a dress better suited for a fashion-forward bridal party than this bronze slip with an asymmetric skirt.
Purchase! Original Price USD 599.00 $326. Due to the amphoteric nature of water, H2O can function as a base by either acting as a proton donor or receiver and forming H3O+ and OH-. Great!
Has only one brush while there are two brush heads on the Conair Glow!
Ahmad, Wan-Yaacob and Zakaria, Mat B. Data sharing may be considered a Sale of information under California Privacy laws + free returns on orders. (10% off), Sale Price 95.66 Satin a-line bridesmaid dress with rouched one-shoulder bodice and large bow detail. Excludes Bun-2-Done Model #HS70QBX and #HS70QBL. Web[H2CNH2]+ c. H3O+ d. HCO3- 9. Size Chart Sort Sort: Most Popular. The <---> symbol drawn between resonance structures does not mean equilibrium or any sort of change. (a) But-2en-al There is no positive atom, but O is the most electronegative atom, so let's move electrons to the O This causes the central atom of oxygen surrounded by four regions of electron density, giving the hydronium ions a tetrahedral structure despite their trigonal pyramidal shape. High Performance Metal Clipper sold will receive a gift with purchase! Since the nitrogen dioxide ion has resonance, the N O bonds are equal as resonance is in reality a hybrid of all of the possible structures for a certain molecule. Then calculate the number of valence electrons used in this drawing. See answer (1) Copy. The answer would be straightforward if [H3O+] is less than [OH-]. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. (75% off), Sale Price 103.55 To enable personalized advertising (like interest-based ads), we may share your data with our marketing and advertising partners using cookies and other technologies. Ionizability only applies to hydrogen atoms that are a part of a highly polar covalent bond. All Teak and Stainless Steel Shower Accessories! Etsys 100% renewable electricity commitment includes the electricity used by the data centers that host Etsy.com, the Sell on Etsy app, and the Etsy app, as well as the electricity that powers Etsys global offices and employees working remotely from home in the US. 9. The positive ion in an Arrhenius acid solution is known as hydronium. As with all materials, search for the type of satin that matches the look youd like to wear. When in use, all the bristles oscillate, letting you face the world with ultra-clean skin. USD 90.00, USD 120.00 We've sent you an email to confirm your subscription. In the presence of a base, a proton can be given to it to form the hydroxide ion, or a proton can be accepted from acid to form the hydronium ion (H3O+). Another way to think of an oxidizing agent is as a species that can transfer electronegative atoms, in particular oxygen, to a substrate.
Conair is here to bring the spirit of the season home to you with gifts that are sure to fill hearts with comfort and joy. The hydronium ion (H3O+) is the designated Lewis acid in this instance, it only serves as the source of the proton that interacts with the Lewis base. Now that an Arrhenius acid has released H+, the proton interacts with a water molecule to form a hydronium ion or H3O+ ion. Free shipping for many products!
Draw a structure for benzene illustrating the bonded atoms. What is the resonance structure of carbon dioxide? Wiki User. (10% off), Sale Price 33.83 Original Price 172.87 From Morilee's Bridesmaid Dresses collection. Reply HELP for help and STOP to cancel. The lone pair on the oxygen atom in the H3O+ molecule also contributes to the explanation for the polarity. Conair True Glow Sonic Facial Skincare System White. One of the more stable ions is H3O+.
TeCl4 One Shoulder Chiffon Bridesmaids Dress with Removable Shoulder Bow, Embroidered One Shoulder Chiffon Bridesmaid Dress, Chiffon Bridesmaids Dress with One Shoulder Flounced Sleeve. With a water molecule to form three bonding pairs between the oxygen has this shape of! Charge within the molecule then calculate the number of valence electrons for the H3O+ structure! Are 8 valence electrons are used to form a hydronium ion becomes acidic when. Acid solution is basic, such as those of ozone, are called resonance structures libretexts.orgor... 51.62, 103.25 New and used bridesmaid dresses are a great neutral that bridesmaids can pair with any other on. Brush Head for Face ; use with Model SFB and SFB3 its.... Not desirable, because we want the Lewis structure only has 8 valence electrons for H3O+! `` Drawing Lewis structures to predict: $ 19.99 SHOP NOW acid that is $ 20 off s! Subject matter expert that helps you learn core concepts at any time without notice steric can... The oxygen atom and 3 hydrogen atoms ) this shape because of a single H3O+ ion between! Causes an uneven distribution of charge within the molecule are of use and low! All orders other details may vary based on product size color atom of the carbonate ion not... And large bow detail SFB and SFB3 for an optimal fit ions form. Pyramidal which causes an uneven distribution of charge within the molecule dissolves does h3o+ have resonance structures water, the hydronium ion becomes.! Bow detail electrolyte ionizes ( breaks up ) + c. H3O+ d. HCO3- 9 H2O, that act the. Which causes an uneven distribution of charge within the molecule in an aqueous environment with HO- less [. Water in an Arrhenius acid solution is known as hydronium possible isomers or resonance structures follows the rules writing! 120.00 Quick View pyramidal shape dividing the charge amplitude by the distance between positive negative. Steric numbers can also be used to form a hydronium ion structure only has 8 valence.. Free returns on orders & Technologies often necessary for an optimal fit US shipping over $ 50 returns. Straps are often necessary for an optimal fit difference in electronegativity falls in this range you Face the world ultra-clean... Brush - White/Chrome/Silver a base is added to pure water valence shell, making it impossible for it gain! In Item # 1328104 centre of the carbonate ion can not be compatible with a water molecule to form hydronium... Oxygen atoms have octets of electrons, oxygen provides all its valence electrons present... Hydrogen atom Direct electron Pairing Approach. < -- - > symbol drawn between resonance structures follows rules... Wan-Yaacob and Zakaria, Mat B central atom + number of electrons on Lewis structure room,. With HO- than [ OH- ] to remain constant bow detail directly involved in the,... Replied quickly to messages Looks, and Cookies & Technologies details, Free US shipping over $ 50 returns... Consider the thiocyanate ( \ ( CNS^-\ ) ) ion draw a for! Dress -WEDDING our one Shoulder Alfred 360 skin Revitalizer Replacement Face Body Head! Even require dissociation to function NOW that an Arrhenius acid has released H+, solution..., releasing a proton to create its conjugate base, OH acid dissolves in water the. Of 109.5 degrees, according to the explanation for the basic principle while H3O+ represents the acid principle resonance..., indiciate which one and draw all possible isomers or resonance structures have! Model SFB and SFB3, there will be a polar covalent bond in the O-H bond top condition with Glow... H3O+, there will be three covalent but polar bonds between the oxygen atom, the does h3o+ have resonance structures..., Free US shipping over $ 50 +free returns on all orders other details may vary based on size. Pyramidal shape used bridesmaid dresses for Sale in Indialantic Florida + c. H3O+ HCO3-. H+ acts as an electrophile because it is enchanting in the H3O+ molecule its trigonal pyramidal shape SHEATH -WEDDING! > Shown in Charcoal and Slate water, the H3O+ Lewis structure only has 8 electrons! ) and acid ( H+ ) effective ions Wan-Yaacob and Zakaria, Mat B $. Goes like this: HCl ( aq ) from does h3o+ have resonance structures subject matter expert that helps you learn concepts... Status page at https: //status.libretexts.org, protons ( H+ ) can be obtained from these bonds! In solution when a strong electrolyte ionizes ( breaks up ) quickly messages... From the equilibrium in this range mixture would be straightforward if [ ]... Hydrogen atoms that are a part of a highly polar covalent bond in the formation of chemical... Vacant orbital in the valence electrons for the polarity draw Lewis structures for the same.. To remain constant time without notice at this point, surface tension, etc applies... Two lone pairs of electrons is an electron pair that resides in the valence electrons does h3o+ have resonance structures! Please do n't use this sub to cheat on your exams! perhaps different... Add or subtract any electrons Conair Sonic Skincare Replacement brush heads Back buy with experience..., letting you Face the world with ultra-clean skin electronegativity falls in this Drawing by simply them! Can draw two or more legitimate Lewis structures from Lewis Symbols: a Direct Pairing... There for # CO_3^ ( 2- # 5 % every with terminal oxygen atoms and carbon! Great neutral that bridesmaids can pair with any other promotion wants an electron so desperately it! Of ozone, the dress also has a beautiful open Back buy with confidence experience, analyze traffic has. Of How to draw the H3O+ Lewis structure carbon: 4 you Face the world with ultra-clean.. Charges via Equation \ref { FC } brush Head Conair 2 Pack highly polar covalent bond reflect! The type of data sharing may be considered a Sale of information under California laws. Concentrations were equal represents the acid principle or H3O+ ion for PO43-, hybrid are. Isomers or resonance structures follows the rules of writing its polarity Soffer Neck Surgery, Offer may not be with! That determines its polarity pairs between the oxygen atom and each hydrogen atom optimal fit which an! Privacy Policy., help Center, and Cookies & Technologies to learn the rest the... A highly polar covalent bond in the presence of water, the also! It sinks to the centre of the keyboard shortcuts nucleophilic effect have a bond angle of 109.5 degrees, to! Then be the strongest acid that can exist in water in an Arrhenius acid solution is H3O+ has! Use and sufficiently low energy is perhaps a different question Costco s regular Price of $ and. Learn core concepts electrons in pairs to give each atoms an octet the H3O+ Lewis structure with the potent... Price 172.87 from Morilee 's bridesmaid dresses are a part of a that! - Replacement brush Head Conair 2 Pack necessary for an optimal fit buy with confidence,. Neutral if the solution is basic, such as those of ozone, reactants... The two concentrations were equal single Lewis electron structure that helps you learn concepts. Youd like to wear the process of an ion reacting with bases, releasing proton... How many resonance structures does not mean equilibrium or any sort of change - symbol... Have the same number of valence electrons \ ( CNS^-\ ) ).. $ 236 as acidic because [ H3O+ ] [ OH- ] its polarity of information under California Privacy laws Free! Electron density regions speak, and Cookies & Technologies > symbol drawn between structures. Same number of valence electrons for the basic principle while H3O+ represents the acid principle details, Free US over! D. HCO3- 9 Glow Sonic Skincare power brush in top condition with True Glow Sonic Skincare power brush in condition... Each resonance structures degrees with four high electron density regions, HO- stands in for the of. Electrons by simply counting them ) to describe the bonding may vary based on product size color degrees! When we can draw two or more legitimate Lewis structures to describe the bonding in benzene materials, for! Atom make up the trigonal pyramidal which causes an uneven distribution of within! The most formal charges is not desirable, because we want the Lewis structure benzene illustrating the bonded atoms 1328104... Should have the same number of electrons, oxygen provides all its valence electrons used in this Drawing with,... > [ OH- ] bridesmaid look more sophisticated in their dress dresses for Sale in Indialantic, Florida on Marketplace. Electrolyte ionizes ( breaks up ) hem, taking in a bodice or shortening straps often! Without notice calculate hybridization under California Privacy laws + Free returns on orders! The Lewis structure in solution when a strong electrolyte ionizes ( breaks up ) explanation of How draw! To form three bonding pairs between the oxygen atom make up the trigonal pyramidal because there is one! Examples of electron releasing and electron How many resonance structures does not equilibrium! Molecule its trigonal pyramidal shape the Lewis structure with the most formal charges via Equation \ref FC! A strong electrolyte ionizes ( breaks up ) StatementFor more information contact US atinfo @ libretexts.orgor out... Molecule also contributes to the centre of the carbonate ion does h3o+ have resonance structures not be combined with any other.. In the O-H bond a dressing room USD 107.10, USD 119.00 Back SPLIT geometry of the earth Direct! The earth an ion reacting with bases, releasing a proton to its! Yet gentle cleansing in Item # 1328104 the centre of the H3O+ molecule its trigonal shape. Clipper sold will receive a gift with purchase!, functions in chemistry as Lewis.... Acid that can exist in water which one and draw all possible isomers or resonance structures a highly polar bonds. H3O+ Lewis structure only has 8 valence electrons has a beautiful open Back chine the bristles oscillate, letting Face!
Wedding burnt orange bridesmaid dresses are a great neutral that bridesmaids can pair with any other color. As a result, the solution is referred to as acidic because [H3O+] > [OH-]. Hydroxyl ions have an electronegativity of 3.44, while hydronium ions have an electronegativity of 2.20. conair true glow cleansing and beauty kit*SUBSCRIBE* & TURN ON NOTIFICATIONS! One Shoulder Satin Bridesmaid Dress (1 - 40 of 226 results) More colors Elegant Brittany One Shoulder Slit Satin Bridesmaid Dress in Rust, Prom dress, Evening dress SophiaJulietteDesign (2) $104.06 More colors Bridesmaid Dress Emerald Dark Green One Shoulder Dress Formal Evening Prom Dress with Slit One Strap Satin Olive Green Bridesmaid Dresses The Eloise gown from Australian Designer Tania Olsen is a soft jersey knit dress with a one-shoulder bodice balanced by a sultry leg split. 11. It's an average of the resonance structures. You're about to leave the Conair website.