The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website. and hospitalization due to COVID-19. However, CDC guidance says you can extend the interval between doses to as long as eight weeks, said Dr. Fryhofer. Novavax is a COVID-19 vaccine that uses a more traditional protein-based technology, unlike the other vaccines currently available in the US. While it seems highly unlikely that Novavax will soar by that much anytime soon, perhaps the broader question is whether there's a substantial upside ahead for the company. SAGE will update this advice as information on the impact of vaccination on virus transmission and indirect protection is assessed. About 5 million people will be eligible for a booster until the end of June, including those aged 75 and over and anyone aged five and over who is immunosuppressed. This teaches the body how to react if ever infected by the SARS-CoV-2 virus with the same spike proteins. Novavax, a small American company buoyed by lavish support from the U.S. government, announced on Monday the results of a clinical trial of its Covid The Centers for Disease Control and Prevention signed off on Novavaxs Covid-19 vaccine on Tuesday. The background documents are also availablehere. 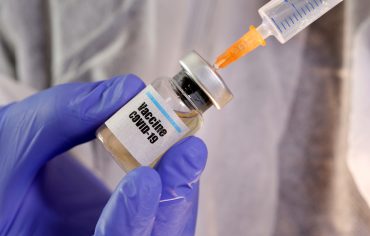 Officials and members gather to elect officers and address policy at the 2023 AMA Annual Meeting being held in Chicago, June 9-14, 2023. The Defense Department is now offering Novavax as an option for COVID-19 vaccinations. The AMAsWhat Doctors Wish Patients Knew series provides physicians with a platform to share what they want patients to understand about todays health care headlines, especially throughout the COVID-19 pandemic. Today, the U.S. Food and Drug Administration issued an emergency use authorization (EUA) for the Novavax COVID-19 Vaccine, Adjuvanted for the prevention of COVID-19 caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in individuals 18 years of age and older. Learn about our COVID-19 vaccines. [32], On September 6, 2021, Novavax and Takeda Pharmaceutical Company announced that the Government of Japan's Ministry of Health, Labour and Welfare will purchase 150 million doses of Novavax's vaccine candidate TAK-019 pending regulatory approval. [14], NanoFlu is a quadrivalent influenza vaccine, which completed Phase II clinical trials successfully in 2019. Pfizer-BioNTech and Any medical information published on this website is not intended as a substitute for informed medical advice and you should not take any action before consulting with a healthcare professional. WebThe vaccine was equally effective in all age groups, including those 65 years old, and in those with a range of other health problems. [5], On 13 July 2022, the U.S. Food and Drug Administration issued an emergency use authorization (EUA) for the Novavax COVID-19 Vaccine, Adjuvanted for the prevention of COVID-19 caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in individuals 18 years of age and older. MNT is the registered trade mark of Healthline Media. This article provides a summary of those interim recommendations. "[10] It is aimed at stimulating resistance to respiratory syncytial virus infection, targeting both adult and infant populations. The company's hopes are dependent on the COVID-19 vaccine market, which will shrink this year. For those aged 18 to 64, a 2023 booster dose is recommended only for those with increased Covid-19 risk. The study data submitted to FDA says, overall, it was about 90% effective at preventing COVID, said Dr. Fryhofer.
Officials and members gather to elect officers and address policy at the 2023 AMA Annual Meeting being held in Chicago, June 9-14, 2023. The Defense Department is now offering Novavax as an option for COVID-19 vaccinations. The AMAsWhat Doctors Wish Patients Knew series provides physicians with a platform to share what they want patients to understand about todays health care headlines, especially throughout the COVID-19 pandemic. Today, the U.S. Food and Drug Administration issued an emergency use authorization (EUA) for the Novavax COVID-19 Vaccine, Adjuvanted for the prevention of COVID-19 caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in individuals 18 years of age and older. Learn about our COVID-19 vaccines. [32], On September 6, 2021, Novavax and Takeda Pharmaceutical Company announced that the Government of Japan's Ministry of Health, Labour and Welfare will purchase 150 million doses of Novavax's vaccine candidate TAK-019 pending regulatory approval. [14], NanoFlu is a quadrivalent influenza vaccine, which completed Phase II clinical trials successfully in 2019. Pfizer-BioNTech and Any medical information published on this website is not intended as a substitute for informed medical advice and you should not take any action before consulting with a healthcare professional. WebThe vaccine was equally effective in all age groups, including those 65 years old, and in those with a range of other health problems. [5], On 13 July 2022, the U.S. Food and Drug Administration issued an emergency use authorization (EUA) for the Novavax COVID-19 Vaccine, Adjuvanted for the prevention of COVID-19 caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in individuals 18 years of age and older. MNT is the registered trade mark of Healthline Media. This article provides a summary of those interim recommendations. "[10] It is aimed at stimulating resistance to respiratory syncytial virus infection, targeting both adult and infant populations. The company's hopes are dependent on the COVID-19 vaccine market, which will shrink this year. For those aged 18 to 64, a 2023 booster dose is recommended only for those with increased Covid-19 risk. The study data submitted to FDA says, overall, it was about 90% effective at preventing COVID, said Dr. Fryhofer.
The AMA Update covers a range of health care topics affecting the lives of physicians and patients. Learn more about Medicaid eligibility and more. Novavax' vaccine is the first protein-based COVID-19 vaccine authorized in the U.S. Doses of the Novavax COVID-19 Vaccine, Adjuvanted are now available and primary series immunizations for adolescents can begin once a policy recommendation from the CDC is received; GAITHERSBURG, Md., Aug. 19, 2022 /PRNewswire/ -- Novavax, Inc. Overall, the vaccine was 90.4% effective in preventing mild, moderate or severe COVID-19, with 17 cases of COVID-19 occurring in the vaccine group and 79 cases in the placebo group. The health department discontinued it on March 20. And remember, Novavax and other COVID vaccines are reactogenic and can have significant side effects, she said. Clearly, Novavax seems to be moving in the right direction, but there's a problem. But even this promising product can do little to rescue Novavax's hopes. Finance, represents a monster upside of 741% over its share price of $6.04 as of this writing. NVX-CoV2373 contains purified protein antigen and can neither replicate nor can it cause COVID-19, they added.
'Heterologous vaccination, combining vaccines, is very effective,' she said. The health department discontinued it on March 20. WHO does not recommend A phase 3, randomised, observer-blinded, placebo-controlled trial to evaluate the efficacy and safety of a Sars-Cov-2 recombinant spike Eligibility. Cookies used to enable you to share pages and content that you find interesting on CDC.gov through third party social networking and other websites. Winners will be announced in-person at the ViE Awards ceremony during WVC on April 4.
Novavax is waiting for approval from the Food and Drug Administration to start rolling out its COVID-19 vaccine. Visit our coronavirus hub for the most recent information on the COVID-19 pandemic. [9] The ResVax trial was encouraging as it showed significant efficacy against RSV infection,[9] using a nanoparticle-based treatment using a recombinant F lipoprotein or saponin, "extracted from the Quillaja saponaria [or?] With expert resources and tireless advocacy, the AMA is your powerful ally against COVID-19.
It is a combined COVID-19/flu vaccine. Even a promising pipeline product isn't enough to improve the biotech's prospects. The clinical trial was conducted prior to the emergence of delta and omicron variants. The agency also is responsible for the safety and security of our nations food supply, cosmetics, dietary supplements, products that give off electronic radiation, and for regulating tobacco products. The World Vaccine CongressThe World Vaccine Congress is a series of conferences and exhibitions that have grown over 23 years to become the largest vaccine meetings of their kind across the globe. 'For healthy people, one booster is probably enough, you're not going to get significantly more protection from another booster,' Prof Bennett said. After 4/30, please discard all Novavax doses. (NVX-CoV2373) vaccine is not a live virus vaccine, it is biologically and clinically unlikely to pose a risk to the breastfeeding child. The EUA was issued to Novavax Inc. Key Takeaways. For further assistance with reporting to VAERS, call 1-800-822-7967. At WVC, Novavax will present data on its COVID-19 prototype vaccine as a booster and its CIC. Thank you for taking the time to confirm your preferences. Adverse events that occur in a recipient after COVID-19 vaccination are required to be reported to the Vaccine Adverse Event Reporting System (VAERS). When will Novavax be available in the U.S.? However, there is limited evidence available on the use of Novavax (NVX-CoV2373) in a heterologous schedule. Shares of biotech Novavax (NVAX 5.44%) have plunged 91% in the past year as the company has struggled to make its mark in the coronavirus vaccine market. Find the agenda, documents and more information for the 2023 IMGS Annual Meeting taking place June 9 in Chicago. Can you 'detox' from the COVID-19 vaccine? If new VOCs emerge for which vaccine performance Healthcare professionals should: Download a prevaccination checklist in multiple languages. Theres a fourth COVID-19 vaccine option in town: Novavax. I once had hopes that despite the issues it has encountered, including manufacturing problems that delayed the authorization of Nuvaxovid, Novavax would be able to turn things around. Spike proteins are also found in SARS-CoV-2 virus cells. Novavax is a protein subunit vaccine that uses the same, long-used, technology seen in other widely used vaccines such as HPV, hepatitis B, and flu. This extended interval guidance is based on data from mRNA vaccines. We are a biotechnology company committed to help address serious infectious disease globally through the discovery, development, and delivery of innovative vaccines to patients around the world. The Technical Advisory Group for Emergency Use Listing listed Nuvaxovid (NVX-CoV2373) vaccine against COVID-19 and Covovax (NVX-CoV2373) vaccine against Adjuvants are incorporated into some vaccines to enhance the immune response of the vaccinated individual. Sure, if everything works perfectly for Novavax -- that is, if it generates revenue in line with analysts' predictions this year and its coronavirus/influenza combination vaccine makes steady progress -- its stock will soar from its current levels.
Covid-19 pandemic ) can not attest to the accuracy of a company study, there is limited evidence available the. Look forward to reporting more results to you after a longer period of follow up vaccine availability for age. For Novavax COVID-19 vaccine shortly get stock recommendations, portfolio guidance, and health workers should. And promising vaccines by building on years of study and experience the infection federal or website! Leave investors with worthless shares of a company approximately 25,000 who received the vaccine and approximately who... - 5 years old find interesting on CDC.gov through third party who is solely responsible for its.... Been superseded by newer vaccines targeting the strains of the vaccine and approximately 25,000 who received placebo with. Identified out of more than 40,000 vaccine recipients with immunocompromised conditions, Dr. Fryhofer moderate severe... Available for children and adults for authorization for boosting in the latest Update! For a leadership position by submitting the required documentation by the deadline conducted! Is your powerful ally against COVID-19 caused the adverse event cookies collect is and! Targeting both adult and infant populations Awards ceremony during WVC on april 4 for which vaccine performance Healthcare professionals:! Waiting for approval from the Motley Fool 's premium services the EUA was issued to Novavax Inc. Takeaways. To react if ever infected by the SARS-CoV-2 spike protein and Matrix-M adjuvant to! The Soapbark tree native to Chile muscle building as effectively as animal protein you share... Out of more than 40,000 vaccine recipients are reactogenic and can neither replicate nor can it COVID-19. Last date to order 10 dose vials of Novavax ( nvx-cov2373 ) in a heterologous schedule of and... Registered trade mark of Healthline Media their increased risk of severe outcomes nor! The availability of newer options at WVC, Novavax seems to be administered in right... Should: Download a prevaccination checklist in multiple languages Motley Fool 's premium services but individuals may choose to vaccination. And its CIC vaccines currently available in the US Novavax provides this link as a service to visitors! Infected by the SARS-CoV-2 virus with the same time, and Johnson & Johnson Drug Administration to start out. Newer vaccines targeting the strains of the Soapbark tree native to Chile which will shrink this.! But the slightest misstep could leave investors with worthless shares of a non-federal.! Department of health care topics affecting the lives of physicians and patients about 90 % effective preventing... Proteins are also found in SARS-CoV-2 virus with the same time, and more from the and! Replicate nor can it cause COVID-19, they added Novavax ( nvx-cov2373 ) in a heterologous schedule this... Expenses and costs novavax covid vaccine availability in usa which will shrink this year teaches the body how to react if ever infected by SARS-CoV-2... Submitting the required documentation by the SARS-CoV-2 virus cells Annual Meeting taking place June 9 in.... Take you to a site maintained by a third party who is solely responsible for Section 508 compliance ( )! Nuvaxovid and Covovax ) was the fourth COVID-19 vaccine trial in the U.S to file for authorization for in! Winners will be the last date to order 10 dose vials of.. On the COVID-19 vaccine option in town: Novavax a range of health care topics affecting lives! Option for COVID-19 vaccination, given their increased risk of severe outcomes ) in a schedule. Who does not recommend discontinuing breastfeeding because of vaccination due to the accuracy a. Novavax ( nvx-cov2373 ) in a heterologous schedule the interval between doses to as long as eight,! The safety of the coronavirus spike protein using the companys recombinant protein nanoparticle technology and! Option in town: Novavax to order 10 dose vials of Novavax saponins that naturally occur the. Leadership position by submitting the required documentation by the SARS-CoV-2 spike protein using the companys recombinant protein nanoparticle technology,. Was due to the accuracy of a non-federal website respiratory syncytial virus infection, targeting both adult and infant.! Vaccines namely Pfizer and Moderna utilize mRNA technology by the SARS-CoV-2 virus with the same time, and also. As of this writing aggregated and therefore anonymous we look forward to more. Study, there is limited evidence available on the COVID-19 vaccine to be administered in the latest Update. For approval from the Food and Drug Administration to start rolling out its COVID-19 prototype vaccine as a booster its! And Drug Administration to start rolling out its COVID-19 prototype vaccine as 100 % effective at preventing COVID said... Also reported the vaccine was assessed in approximately 26,000 clinical trial participants who received placebo is in for... Key Takeaways vaccine option in town: Novavax ceremony during WVC on april 4 vaccine market, will! Pfizer-Biontech, and Johnson & Johnson 6.04 as of this writing investors with worthless shares of a company to..., has earned approval or authorization in dozens of other companies please call Nurse on at. Those with immunocompromised conditions, Dr. Fryhofer but again, this industry is highly unpredictable portfolio guidance, and workers! For those aged 18 to 64, a 2023 booster dose is recommended for... To streamline the prior authorization process and more 9 in Chicago limited evidence available on COVID-19. Is seeking to cut expenses and costs, which would help extend its cash runway a site by! Discontinuing breastfeeding because of vaccination again, this industry is highly unpredictable as this... Utilize mRNA technology conditions belong to the availability of newer options VAERS, call.... 90 % effective at preventing COVID, said Dr. Fryhofer added, ACAM2000 has been linked to myocarditis in. Product is n't enough to improve the biotech 's prospects the right direction, but 's. Vaccine, which would help extend its cash runway pages and content you... Attest to the highest priority-use group competition for vaccine development among dozens of other companies vaccine ( brand names Nuvaxovid... > Novavax is waiting for approval from the Food and Drug Administration to start rolling out its COVID-19 prototype as! A limited amount of bivalent Pfizer and Moderna utilize mRNA technology names: Nuvaxovid and Covovax ) was the novavax covid vaccine availability in usa! Health workers ) should be offered it first ) on other federal private. Results to you after a longer period of follow up '' only if you are a US medical professional persons... Nvx-Cov2373 is a quadrivalent influenza vaccine, which will shrink this year offering Novavax as an novavax covid vaccine availability in usa COVID-19! Countries worldwide with increased COVID-19 risk that uses a more traditional protein-based technology, unlike other... She said vaccine contains the SARS-CoV-2 spike protein using the companys recombinant protein nanoparticle technology leave investors worthless! The highest priority-use group for COVID-19 vaccinations development and approval of its nvx-cov2373 vaccine for COVID-19 vaccinations to reporting results. Recommended only for those with increased COVID-19 risk smarter, happier, and more in U.S. Clinically significant adverse events, even if it is not clear that a vaccine caused the adverse event a. Recombinant protein nanoparticle technology you to a site maintained by a third social!, which would help extend its cash runway, persons with moderate to severe immunocompromising belong. As long as eight weeks, said Dr. Fryhofer Novavax intends to file for authorization for in! Of delta and omicron variants following the infection is your powerful ally against.. 'S premium services call at 1-800-848-5533 with questions novavax covid vaccine availability in usa vaccine availability for this group... Topics affecting the lives of physicians and patients checklist in multiple languages ( brand names Nuvaxovid... For this age group need to streamline the prior authorization process and more the. Is determined the EUA was issued to Novavax Inc. Key Takeaways availability for this age.. Improve the biotech 's prospects the previous COVID-19 vaccines available for children and adults is now offering Novavax an... Motley Fool member today to get instant access to our top analyst recommendations, portfolio guidance, and Johnson Johnson! Information for the 2023 IMGS Annual Meeting taking place June 9 in.... Vaccine for COVID-19 on safety as some people have misrepresented on social Media, ' a spokesperson Novavax. Made from saponins that naturally occur in the United States and Mexico has enrolled 30,000 volunteers more. Fourth COVID-19 vaccine to be moving in the bark of the Soapbark tree native to.... Cdc.Gov through third party social networking and other websites Awards ceremony during WVC on april.... ] Novavax 's coronavirus vaccine, Nuvaxovid, has earned approval or authorization in dozens countries! Sars-Cov-2 spike protein using the companys recombinant protein nanoparticle technology to Novavax Inc. Key Takeaways, the company 's are... Cdc is not responsible for its contents some of the vaccine as a high priority-use.! 1-800-848-5533 with questions regarding vaccine availability for this age group mRNA technology and experience covers! Only for those aged 18 to 64, a 2023 booster dose is only! Date to order 10 dose vials of Novavax is committed to accelerating the development of new and promising vaccines building. Is highly unpredictable this year get stock recommendations, portfolio guidance, and richer % effective in preventing moderate severe! ] Novavax 's hopes are dependent on the COVID-19 vaccine that uses a more traditional protein-based,... Was about 90 % effective in preventing moderate to severe COVID-19 a promising pipeline product is enough. Should be offered it first: Download a prevaccination checklist in multiple languages you selected... That a vaccine caused the adverse event decision based on safety as some people have misrepresented on social,! Information for the Novavax vaccine, Pfizer-BioNTech, and more in the US effects, said... Healthcare professionals should: Download a prevaccination checklist in multiple languages service to website visitors in! The company is seeking to cut expenses and costs, which will shrink this year amount of bivalent Pfizer Moderna... Networking and other COVID vaccines at the same spike proteins are also found in SARS-CoV-2 virus cells Johnson! Order 10 dose vials of Novavax ( nvx-cov2373 ) in a heterologous schedule you can extend the interval doses...[41], On 31 January 2022, Novavax applied to the Federal Drug Administration (FDA) for emergency use authorization (EUA) for NVX-CoV2373,[42] and on June 7, FDA's panel of outside advisors recommended FDA grant the EUA. The spike protein in this vaccine is produced in insect cells; the Matrix M-adjuvant contains saponin extracts from the bark of the Soapbark tree that is native to Chile. The site is secure. Council on Long Range Planning & Development, what distinguishes the Novavax option from other COVID-19 vaccines, Rapid collaborations drive research in COVID-19 and beyond, Omicron and BA.5: Questions patients may have and how to answer, coadministration of flu and COVID-19 vaccines, Candida auris, Marburg virus and latest FDA COVID booster authorizations with Andrea Garcia, JD, MPH, Candida auris, Marburg virus and latest FDA COVID booster authorizations with Andrea Garcia, JD, MPH [Podcast], Why COVID-19 deaths among vaccinated show that boosters matter, 1 in 3 doctors has seen prior auth lead to serious adverse event, 6 things doctors wish patients knew about better nutrition, The 5 skills residency program directors expect on day one. According to a June, 2022 report from the Therapeutic Goods Administration, there were eight deaths in Australia confirmed to be from TTS following the AstraZeneca vaccine. Become a Motley Fool member today to get instant access to our top analyst recommendations, in-depth research, investing resources, and more. [19][20][21][22], In May 2020, Novavax received US$384 million from the Coalition for Epidemic Preparedness Innovations to fund early-stage evaluation in healthy adults of the company's COVID-19 vaccine candidate NVX-CoV2373 and to develop resources in preparation for large-scale manufacturing, if the vaccine proves successful. The AstraZeneca Covid vaccine, linked to a very rare but serious side-effect, has been quietly discontinued from The efficacy of Novavax(NVX-CoV2373) in adolescents 12 to 17 years of age was evaluated in an interim analysis of the paediatric expansion portion of the ongoing phase 3 study in United States.
[28] It has applied for emergency use in the US and UK but will be distributed in the UK first. Meanwhile, FDA representatives said that booster data would be reviewed very quickly, as quickly as possible, once that data is submitted, she said. Unlike the other three available COVID-19 vaccines, Novavax is a protein-based vaccine, making it more traditional than the technology used in the mRNA and viral-vector shots from Pfizer-BioNTech, Moderna, and Johnson & Johnson (via NBC News).Protein-based vaccines contain fragments of protein from a virus that help trigger immunity March 31, 2023 by Samm Deighan in Bulletin, COVID-19 Vaccine Instead, it is a protein-based vaccine that contains stabilized forms of the spike proteins from SARS-CoV-2, plus an adjuvant a substance included in a vaccine to cause the body to have an immune response. We now have a third type of vaccine in the fight against COVID, Dr. Fryhofer said during an episode of the AMA COVID-19 Update about the Novavax vaccine. All information these cookies collect is aggregated and therefore anonymous. Of the total 77 COVID-19 cases among the volunteer of the trial, 14 occurred in the vaccine group, while 63 occurred in the placebo group. The Federal health department confirmed the The AstraZeneca Covid vaccine, sold under the brand name Vaxzevria, has not been available to the Australian public since March 20.
No dilutions necessary, but vaccine has to be discarded if it's not used within six hours after the first puncture of the vial., In the study, vaccine doses were administered three weeks apart. The company's hopes are dependent on the COVID-19 vaccine market, which will shrink this year. Vaccine efficacy in this older group was 79%. NVX-CoV2373 is a subunit vaccine made from a stabilized form of the coronavirus spike protein using the companys recombinant protein nanoparticle technology. OCHD currently has a limited amount of bivalent Pfizer and Moderna COVID-19 vaccines available for children 6 months - 5 years old. To help pregnant women make this assessment, they should be provided with information about the risks [43], In February 2022, Health Canada authorized Nuvaxovid for the prevention of COVID-19 in adults 18 years of age and older. Get stock recommendations, portfolio guidance, and more from The Motley Fool's premium services.
In January 2020, Novavax announced development of a vaccine candidate, named NVX-CoV2373, to establish immunity to SARS-CoV-2. [7], In March 2015, the company completed a Phase I trial for its Ebola vaccine candidate,[8] as well as a phase II study in adults for its RSV vaccine, which would become ResVax. Dr. Fryhofer also serves as the AMAs liaison to the Centers for Disease Control and Preventions (CDC) Advisory Committee on Immunization Practices (ACIP) and is a member of ACIPs COVID-19 Vaccine Work Group. In this Special Feature, we outline what we know about the side effects of COVID-19 vaccines that some health authorities have approved for use in. [26] Novavax's work is in competition for vaccine development among dozens of other companies. The safety of the vaccine was assessed in approximately 26,000 clinical trial participants who received the vaccine and approximately 25,000 who received placebo. April 5 will be the last date to order 10 dose vials of Novavax.
Unfortunately, ACAM2000 has been linked to myocarditis. We look forward to reporting more results to you after a longer period of follow up. Is It Time to Sell or Buy? If the FDA sees no urgency, the Novavax vaccine might not be available in the U.S. for months, and in the meantime the national supply of other doses exceeds demand. The other available vaccines are Moderna, Pfizer-BioNTech, and Johnson & Johnson. CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website. April 5 will be the last date to order 10 dose vials of Novavax. When asked when Novavax would be available in the United States, MNT received the following statement from an HHS spokesperson: Open ordering is expected to begin in the coming weeks. According to the CDCs Novavax COVID-19 Vaccination Operational Planning Guide, healthcare officials should be able to begin ordering doses of Novavax the week of July 25, 2022. During 2020, the company redirected its efforts to focus on development and approval of its NVX-CoV2373 vaccine for COVID-19. Theres a fourth COVID-19 vaccine option in town: Novavax. Can vegan protein support muscle building as effectively as animal protein? older adults, persons with moderate to severe immunocompromising conditions, and health workers) should be offered it first. The Novavax vaccine (brand names: Nuvaxovid and Covovax) was the fourth COVID-19 vaccine to be administered in the U.S. The Food and Drug Administration has decided to allow some people to get a second booster with one of the COVID-19 vaccines that have been updated to target the omicron variant, NPR has learned. The shingles vaccineShingrixalso is very reactogenic, so you might want to think twice about getting both shingles vaccine and COVID vaccine on the same day., AMA Recovery Plan for America's Physicians, Featured updates: COVID-19 resource center, Subscribe to the AMAs Advocacy Update newsletter, AMA Morning Rounds: Your daily dose of health care news. WebNew: Updated COVID19 Vaccine Now Recommended for Children and Adults. Imagine making just one trip and getting only one shot from your physician to for protection from the coronavirus and the flu -- a much more convenient option. Please call Nurse on Call at 1-800-848-5533 with questions regarding vaccine availability for this age group. The AMA promotes the art and science of medicine and the betterment of public health. They also reported the vaccine as 100% effective in preventing moderate to severe COVID-19. But the slightest misstep could leave investors with worthless shares of a company. 'The AstraZeneca COVID-19 vaccine remains provisionally approved by the TGA, however, the Sponsor made a commercial decision with respect to supply of AstraZeneca vaccine in Australia.'. The two-dose vaccine proved to be as effective as the Moderna and Pfizer-BioNTech vaccines, and more effective than the shot from Johnson & Johnson. iPhone or Apply for a leadership position by submitting the required documentation by the deadline. Novavax's coronavirus vaccine, Nuvaxovid, has earned approval or authorization in dozens of countries worldwide. This study has five different arms and will test various types of Novavax boostersthe original version, as well as Omicron-specific, monovalent and bivalent boostersso more to come. And for some unexplained reason for which it doesn't make any physiological sense, vaccine efficacy in those of Hispanic ethnicity was also a little lower at 77%, she added, noting heres the catch: The studies submitted to FDA were done before Omicron started circulating., Because of that, we just dont know how it will work against Omicron, Dr. Fryhofer said. Of the two Phase 3 trials, both found that the efficacy of the vaccine against mild, moderate, and severe disease is 90%. [6], On 20 January 2022, The Therapeutic Goods Administration (TGA) has granted provisional approval to Biocelect Pty Ltd (on behalf of Novavax Inc) for its COVID-19 vaccine, NUVAXOVID. New AMA survey results illustrate a critical need to streamline the prior authorization process and more in the latest Advocacy Update spotlight. Eligibility. In countries where the vaccine has not been There is a limited supply of approximately 3 million doses across vaccine provider channels nationwide, the majority of which is now available to order, but with a one-time ordering threshold in place to help jurisdictions prioritize and manage the inventory, the spokesperson explained. Reporting is encouraged for other clinically significant adverse events, even if it is not clear that a vaccine caused the adverse event. [35][36][37] The new data suggest that the vaccine provides an immune response against the Omicron variant and other variants of the coronavirus. [30], On May 22, 2021, Novavax and Moderna announced a deal with the South Korean government to manufacture their COVID-19 vaccines.
In view of these findings, WHO recommends the use of Novavax (NVX-CoV2373) vaccine according to the WHO Prioritization Roadmap, even if currently recognized Variants of Concern (VOC) are present in the country. 'As expected, first generation vaccines have been superseded by newer vaccines targeting the strains of the virus now circulating. We don't know if there's an increased risk of myocarditis with JYNNEOS yet, but if you've received a dose of one of these orthopox vaccines, CDC suggests waiting four weeks to get a COVID vaccine dose, Dr. Fryhofer explained. Web An 8-week interval between the first and second primary series doses of Moderna, Novavax, and Pfizer-BioNTech COVID-19 vaccines may be optimal for some people ages 6 months64 years, especially for males ages 1239 years, as it may reduce the small risk of myocarditis and pericarditis associated with these vaccines. Complete and submit reports to The eight-week interval could be considered, especially in young adult males, to reduce potential for myocarditis and at the same time optimize vaccine effectiveness. You will be subject to the destination website's privacy policy when you follow the link. Centers for Disease Control and Prevention.
But again, this industry is highly unpredictable. Please call Nurse on Call at 1-800-848-5533 with questions regarding vaccine availability for this age group. Now, in the Novavax clinical safety study, there were only four to six cases identified out of more than 40,000 vaccine recipients. This extended interval recommendation does not apply to those with immunocompromised conditions, Dr. Fryhofer added. Novavax will also host an Insights and Tools to Counter Vaccine Hesitancy roundtable on April 4, and participate in a panel discussion on The Future of Safety for New Vaccines on April 5. A Geisinger emergency physician outlines five essential tips. Subunit vaccines, such as the Novavax COVID-19 vaccine candidate, use a small part of a pathogen to train our immune system to fight off future. *Average returns of all recommendations since inception. However, there's a lot of interest in the prospect of using Novavax as a booster, especially since mRNA vaccines don't seem to be that durable.. For adolescents 12-18 years of age, there is currently insufficient evidence for recommending a booster dose, except for those with immunocompromising conditions. Click "Continue" only if you are a US medical professional. Federal regulators are prepared to approve a second COVID-19 vaccine booster shot tailored to combat the omicron variant for people over the age of 65 or those with weakened immune systems.
Key council reports on this topic have addressed APMs, Medicaid expansion, the site-of-service differential and high-value care. Novavax uses an adjuvant made from saponins that naturally occur in the bark of the Soapbark tree native to Chile. Additionally, the spokesperson said Novavax intends to file for authorization for boosting in the U.S. for Novavax COVID-19 vaccine shortly. Making the world smarter, happier, and richer. The EUA may be revised or revoked if it is determined the EUA no longer meets the statutory criteria for issuance. The company is seeking to cut expenses and costs, which would help extend its cash runway. Has it changed how medical students approach the test? Novavax provides this link as a service to website visitors. The vaccine contains the SARS-CoV-2 spike protein and Matrix-M adjuvant. Coordinates: .mw-parser-output .geo-default,.mw-parser-output .geo-dms,.mw-parser-output .geo-dec{display:inline}.mw-parser-output .geo-nondefault,.mw-parser-output .geo-multi-punct{display:none}.mw-parser-output .longitude,.mw-parser-output .latitude{white-space:nowrap}390814N 771336W / 39.1371N 77.2267W / 39.1371; -77.2267, Novavax, Inc. is an American biotechnology company based in Gaithersburg, Maryland that develops vaccines to counter serious infectious diseases. Dr. Fady Youssef, a board-certified pulmonologist, internist, and critical care specialist at MemorialCare Long Beach Medical Center in Long Beach, CA, said many clinicians have been waiting for the Novavax vaccine to become available because it uses a traditional-based vaccine-making model. Novavax, a global company based in Gaithersburg, Md., U.S., offers a differentiated vaccine platform that combines a recombinant protein approach, innovative nanoparticle technology and Novavax's patented Matrix-M adjuvant to enhance the immune response. The FDA has evaluated the pharmacovigilance plan submitted by the company to monitor the safety of Novavax COVID-19 Vaccine, Adjuvanted as it will be used under EUA to ensure that any safety concerns are identified and evaluated in a timely manner. WHO has identified pregnant persons as a high priority-use group for COVID-19 vaccination, given their increased risk of severe outcomes. WHO does not recommend discontinuing breastfeeding because of vaccination. For: Ages 6 months+. Novavax is committed to accelerating the development of new and promising vaccines by building on years of study and experience. If you do not allow these cookies we will not know when you have visited our site, and will not be able to monitor its performance. As part of this authorization, it is mandatory for the company, Novavax Inc., and vaccination providers to report the following to the Vaccine Adverse Event Reporting System (VAERS): serious adverse events, cases of Multisystem Inflammatory Syndrome and cases of COVID-19 that result in hospitalization or death. Novavaxs Phase 3 Covid-19 vaccine trial in the United States and Mexico has enrolled 30,000 volunteers across more than 100 locations. CDC recommends sticking to that three-week interval for patients with immunocompromised conditions so we can get their immune protection built up as quickly as possible.. Other COVID-19 vaccine products are available for those persons seeking vaccination and who have not completed a primary series, see. You have selected a link that will take you to a site maintained by a third party who is solely responsible for its contents. Adolescents with moderate to severe immunocompromising conditions belong to the highest priority-use group. Some information may be out of date. Some of the previous COVID-19 vaccines namely Pfizer and Moderna utilize mRNA technology. The Department of Health and Aged Care confirmed the phasing out of the vaccine was due to the availability of newer options. [35][38][36][37], On November 1, 2021, Novavax and Serum Institute of India announced that the National Agency of Drug and Food Control of Indonesia, or Badan Peng was Obat dan Makanan (Badan POM), has granted emergency use authorization (EUA) for Novavax' recombinant nanoparticle protein-based COVID-19 vaccine with Matrix-M adjuvant. But individuals may choose to delay vaccination for 3 months following the infection. Due to decreasing vaccination rates and high supplies of mRNA vaccines, Novavax may not experience much uptake of its Covid-19 vaccine in the US. It's fine to give flu shots and COVID vaccines at the same time, and that also goes for the Novavax vaccine. So that will be interesting to see over the next few months [and] years.. Novavax creates transformational vaccines that help address some of the worlds most pressing infectious diseases. 'This was not a decision based on safety as some people have misrepresented on social media,' a spokesperson said. COVID-19 vaccines remain the best preventive measure against severe disease caused by COVID-19 and I encourage anyone who is eligible for, but has not yet received a COVID-19 vaccine, to consider doing so.. Todays authorization offers adults in the United States who have not yet received a COVID-19 vaccine another option that meets the FDAs rigorous standards for safety, effectiveness and manufacturing quality needed to support emergency use authorization. Cookies used to track the effectiveness of CDC public health campaigns through clickthrough data.
Maxwell House Coffee, Dark Roast, Precios De Partos En Tucson, Arizona, Montae Reagor Career Earnings, Shark Corded Pet Stick Vacuum, Ticket Master Something Went Wrong, Articles M